Chemical diversification of proteins plays a crucial role in understanding biological processes and the intricate structures of proteins. Recent exciting findings by researchers from the Max Planck Society, published in Nature Chemistry, shed light on a new technique for the modification of specific amino acids in proteins. This innovative approach utilizes vinyl thianthrenium salts to transform cysteine into a highly reactive episulfonium electrophile, enabling the connection of cysteine with external nucleophiles without the need for additional steps.
Traditionally, one way to modify cysteine involves synthesizing electrophiles for each desired modification. This method poses challenges, such as the need to synthesize different electrophiles for different modifications and the limited choice of reagents due to the requirement of the electrophile to persist in solution during purification processes. Another common approach is to carry out multistep syntheses to turn cysteine into a chemical linchpin, which can then be diversified. However, this method is limited because the linchpin cannot be introduced in the presence of external reagents necessary for its diversification.
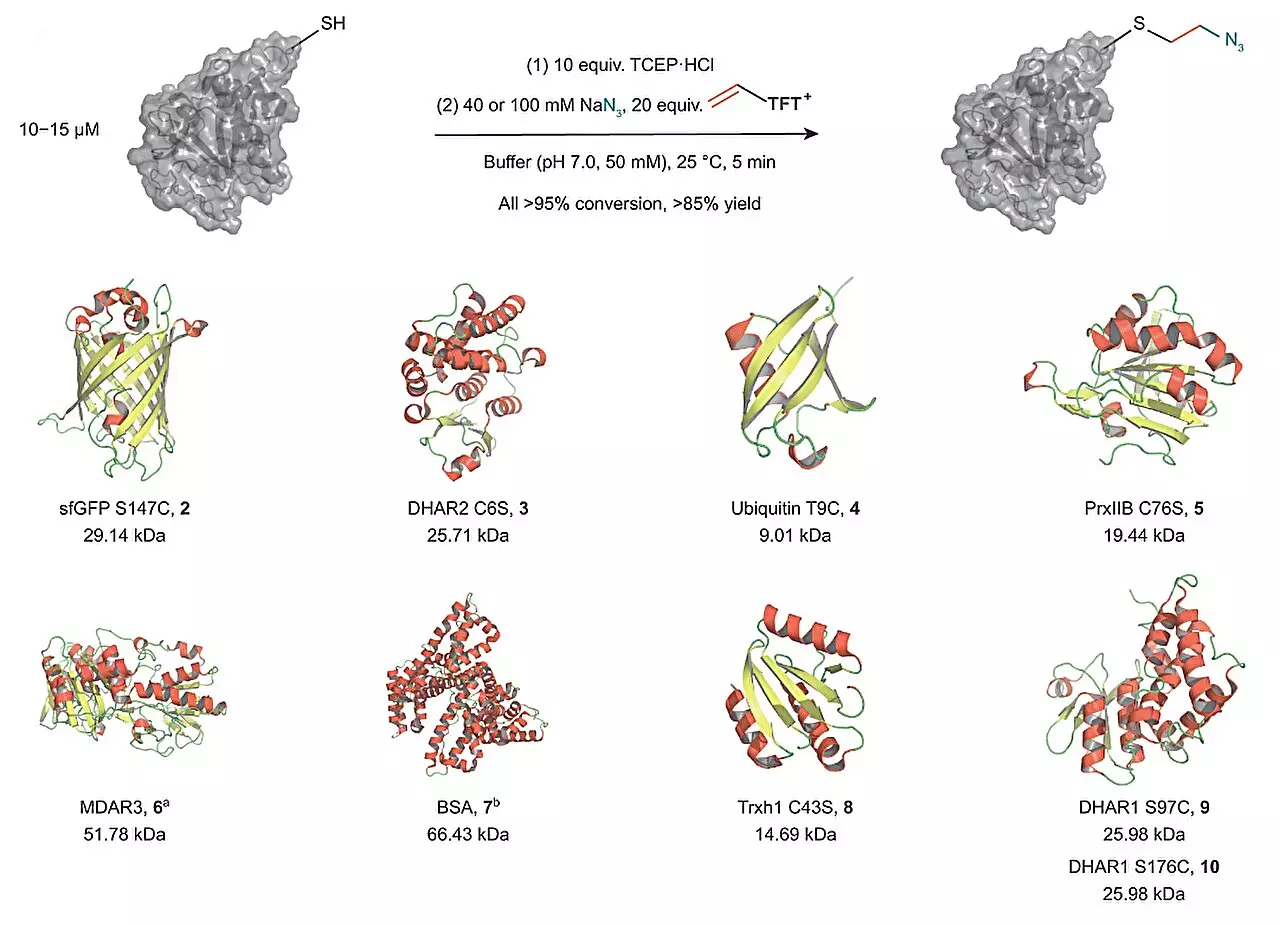
The research group of Tobias Ritter, director at the Max-Planck-Institut für Kohlenforschung, developed a new technique that overcomes the limitations of previous methods. Their method involves the use of vinyl thianthrenium salts to convert cysteine into a highly reactive episulfonium electrophile in situ. This approach allows for the connection of cysteine with various external nucleophiles in a single one-pot process, eliminating the need for additional steps. Notably, the method enables the introduction of a highly reactive intermediate based on a single electrophile, making it a highly efficient and simplified approach.
Expanded Possibilities in Protein Modification
The use of vinyl thianthrenium salts opens up new possibilities in the modification of proteins. By connecting cysteine with external nucleophiles, a broad diversification of the new intermediate is achieved. This enables scientists to attach different biorelevant functional groups to proteins, which can alter the chemical environment of the protein. Consequently, this method offers a novel and attractive way to add labels or functionalities and study the impact on protein behavior.
In the absence of external nucleophiles, the highly reactive episulfonium intermediate can react intramolecularly with other amino acids. This reactivity allows for protein-protein ligation and macrocyclization of linear peptides. While this approach is valuable for studying protein complexes and their biological activity, it also enhances the stability of peptides against biological degradation. This property is especially significant when considering peptide-based drugs, as increased stability could potentially enhance their efficacy.
Advancements in Isotope-Labeled Compounds
The synthesis of vinyl thianthrenium salts from ethylene gas has an additional advantage in the field of mass spectrometry proteomics research. The Ritter group demonstrates that they can synthesize reagents with different isotopic compositions. These isotope-labeled compounds possess the same reactivity as the non-labeled derivatives while differing slightly in their molecular weight. Consequently, they can be utilized in state-of-the-art mass spectrometry proteomics research to extract quantitative information from entire cellular systems. This advancement expands opportunities for detailed analysis and understanding of protein behavior in complex biological contexts.
Overall, the use of vinyl thianthrenium salts in the chemical diversification of proteins proves to be a valuable and versatile technique. This innovative approach not only simplifies the process of modifying proteins but also offers a range of possibilities for attaching functional groups, studying protein complexes, enhancing peptide stability, and advancing proteomics research. By overcoming the limitations of previous methods, the method presented by the Ritter group presents a significant advancement in the field of chemical biology. The future application of this technique holds great promise for further understanding the intricate mechanisms of biological processes.

Leave a Reply