Breast and gynecological cancers continue to be significant health concerns worldwide, causing severe damage to women’s health, fertility, and overall quality of life. However, recent research has provided hope by identifying MYT1 as a potential therapeutic target for these types of cancer. This groundbreaking study, published in the Journal of Medicinal Chemistry, was supported by Insilico Medicine’s AI-driven generative biology and chemistry engine, showcasing the power of artificial intelligence in advancing cancer research.
Through the utilization of Insilico’s proprietary AI-driven target identification platform, PandaOmics, the research team analyzed data on various gynecological cancers, including ovarian, endometrial, cervical, and breast cancer. Notably, MYT1 consistently ranked as a highly relevant target across all diseases. MYT1, a member of the Wee1-kinase family, demonstrates limited expression in normal tissues but is highly expressed in most cancer types. Its inhibition, along with CCNE1 amplification, has been identified as a synthetic lethal therapeutic strategy for cancers with genome instability.
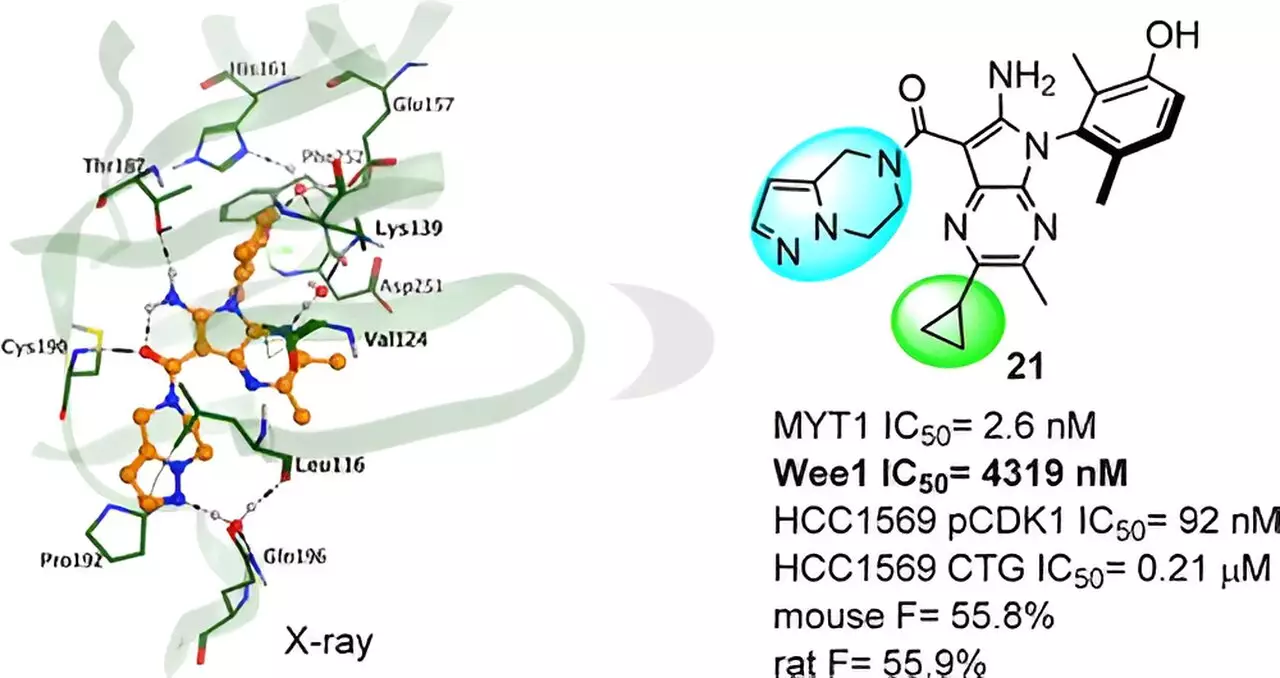
Designing selective inhibitors for MYT1 has been a significant challenge due to its high homology to Wee1. Insilico Medicine addressed this obstacle by leveraging Chemistry42, the AI-driven small molecule generation platform. Employing structure-based drug design (SBDD) strategies, Insilico generated a series of compounds targeting MYT1. Stringent filters for similarity and selectivity were applied, resulting in the emergence of hit compounds. Furthermore, an X-ray crystal structure analysis revealed the impact of subtle chemical modifications on compound activity, offering invaluable insights for further molecular optimization.
From the array of novel compounds, Insilico Medicine discovered the lead compound, Compound 21, through rigorous molecular optimization. Compound 21 exhibited robust MYT1 activity, excellent selectivity over Wee1, and reduced potential for off-target effects when tested against a kinase panel. Moreover, preclinical studies demonstrated Compound 21’s potent in vivo antitumor efficacy and promising profile in ADME (absorption, distribution, metabolism, and excretion) and PK/PD (pharmacokinetics and pharmacodynamics).
“The innovative approach of this program has not only presented a method for effective target identification but has also led to the development of a promising selective MYT1 inhibitor,” stated Yazhou Wang, Ph.D., the medicinal chemistry leader of the MYT1 program at Insilico Medicine and the first author of the published paper. The discovery of Compound 21 and its favorable characteristics open up new avenues for targeted therapy in breast and gynecological cancer. By specifically inhibiting MYT1, researchers can potentially disrupt cell cycle regulation in cancer cells with genome instability, offering a targeted treatment approach with reduced side effects compared to traditional therapies.
The integration of AI-driven technologies, such as Insilico Medicine’s generative biology and chemistry engine, has revolutionized the field of cancer research. The identification of MYT1 as a promising therapeutic target for breast and gynecological cancer, along with the discovery of Compound 21, highlights the potential of precision medicine in combating these devastating diseases. With further research and development, targeted therapies based on selective MYT1 inhibition may soon become a reality, improving the prognosis and quality of life for countless women affected by these cancers.

Leave a Reply