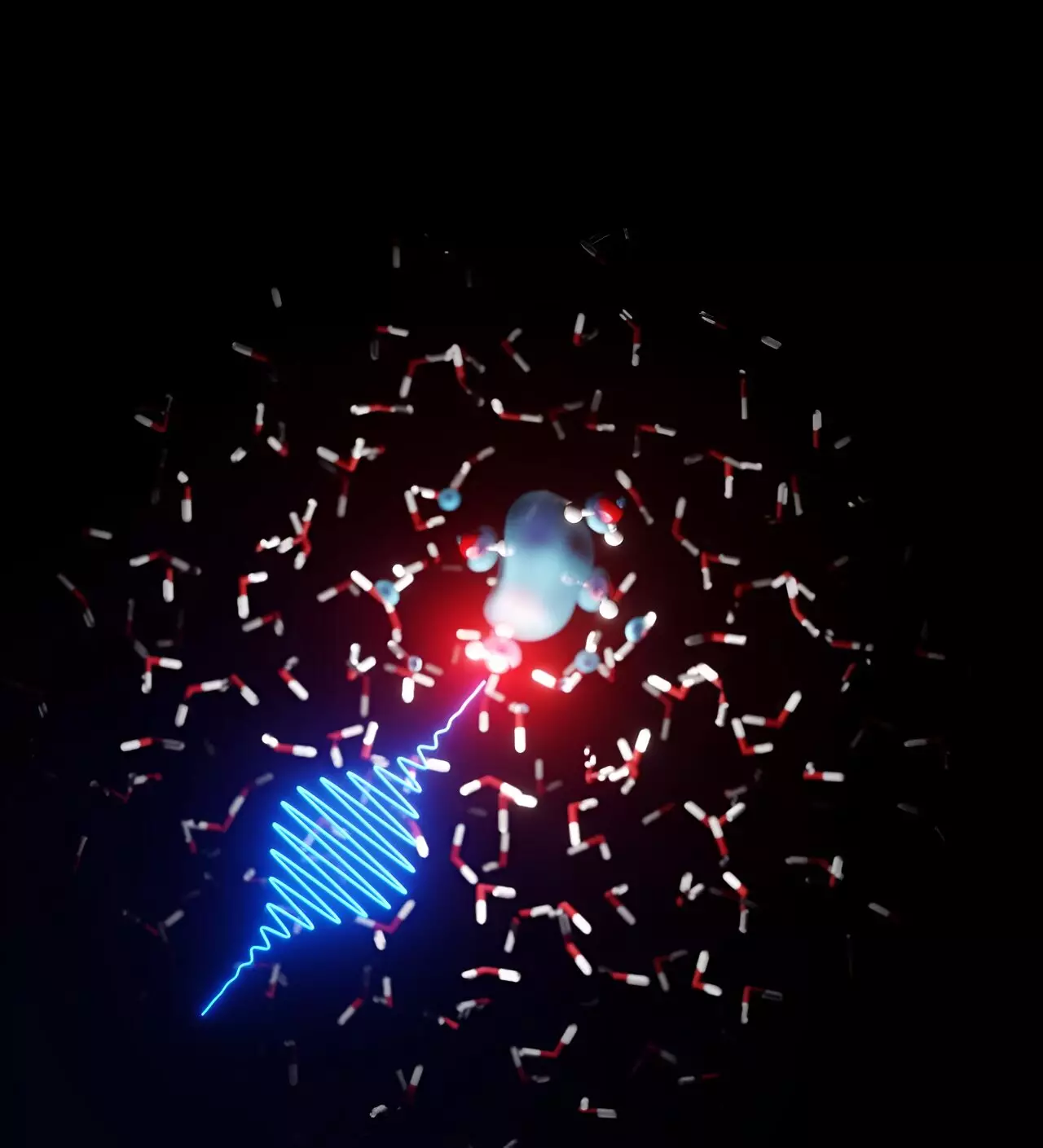
Radiation and its interaction with water have long fascinated scientists and raised important questions about the behavior of free electrons in this crucial substance. In an effort to shed light on this topic, a team of theoretical physicists from DESY collaborated with colleagues from the Argonne National Laboratory in the United States. Using data collected at the LCLS X-ray laser in California, they embarked on a groundbreaking study that may finally resolve a controversy in physics surrounding the presence of free electrons in water and how they behave at extremely short time scales. The results of their research, recently published in the Journal of the American Chemical Society, reveal a stunning discovery: the existence of electron-filled bubbles trapped within cage-like structures amidst individual water molecules.
Free electrons, as the name suggests, are electrons that are not bound to atoms. When radiation interacts with water, some water molecules ionize, resulting in the emergent of free electrons. The pathways through which these electrons flow between water molecules have long been a subject of discussion. The experimental team, led by scientist Linda Young from Argonne, utilized the LCLS X-ray laser to study the behavior of water molecules excited by lasers and imaged by the X-ray laser. Intriguingly, they observed peculiar signatures associated with the excited water molecules. By employing X-ray absorption spectroscopy, they identified structures within these molecules. Seeking a deeper understanding of their discoveries, the experimental team joined forces with a team of theoretical physicists from DESY, led by Ludger Inhester from the Center for Free-Electron Laser Science.
Collaboratively, the scientists uncovered a remarkable finding – free electrons in water form bubble-like structures that are subsequently trapped by water molecules, a process akin to the solvation of chemicals in water at the molecular level. Furthermore, the DESY team elucidated the underlying mechanism of electron solvation in water and its parameters. Their research revealed that as temperature changes, the dissolution process and the subsequent formation of cage structures remarkably fluctuate. Arturo Sopena, the first author of the study, remarks, “It turns out that the dissolution process and thus the formation of the cage structures is remarkably sensitive to temperature changes in the water.”
The newfound insights into the solvation process highlight the intricate dance free electrons perform in water. Initially, these electrons span a wide area among the water molecules before docking onto specific hydrogen bonding patterns within the molecular liquid water structure. Here, they delve into a narrow space, triggering a rapid reorientation of the neighboring water molecules. Astonishingly, this burrowing and reorientation phenomenon concludes within an astonishing 100 femtoseconds, an infinitesimally brief period equivalent to a quadrillionth of a second. The electron-filled bubble, measuring approximately 50 billionths of a meter in width, dissociates within several picoseconds, equal to a trillionth of a second.
Understanding how water reacts when exposed to radiation is of paramount importance, as it plays a crucial role in various disciplines such as radiation chemistry and the interaction of radiation with biological materials. This groundbreaking research offers a critical insight into the initial steps of chemical reactions driven by radiation and the subsequent radiation chemistry. Moreover, the findings open the doors to comprehending the behavior of ionizing radiation-induced damage in water.
The study was conducted as part of the Cluster of Excellence CUI: Advanced Imaging of Matter at Universität Hamburg and provides a stepping stone for further intensive investigations into water-related research. Excitingly, the emerging Center for Molecular Water Science, established through international collaboration on the DESY campus, will dedicate itself to advancing our understanding of the complex interplay between radiation and water.
The collaboration between experimental and theoretical physicists has unveiled a captivating phenomenon – the formation of electron-filled bubbles ensnared within cage-like structures in water. This groundbreaking research deepens our understanding of the intricate behavior of free electrons and their solvation in water, shedding light on the impact of radiation on this crucial substance. The findings carry significant implications for various scientific disciplines and pave the way for further exploration in water-related research.

Leave a Reply