Photocatalysts with highly reducing or oxidizing properties are crucial in the field of photochemistry. The existing transition metal complexes that possess excited state oxidants, such as chromium, iron, and cobalt, necessitate high energy light for excitation and their oxidizing potential remains underutilized. Moreover, these photocatalysts rely on precious and expensive metals, which poses limitations on their widespread application. However, a team of researchers led by Professor Katja Heinze from Johannes Gutenberg University Mainz (JGU) has recently developed a groundbreaking molecular system that utilizes the abundant and inexpensive metal, manganese. This soluble manganese complex exhibits panchromatic absorption and emits near-infrared light, making it an exceptional photocatalyst for a range of organic substrates. These remarkable findings are providing a new direction in photochemistry, as described in a study published in the journal Nature Chemistry.
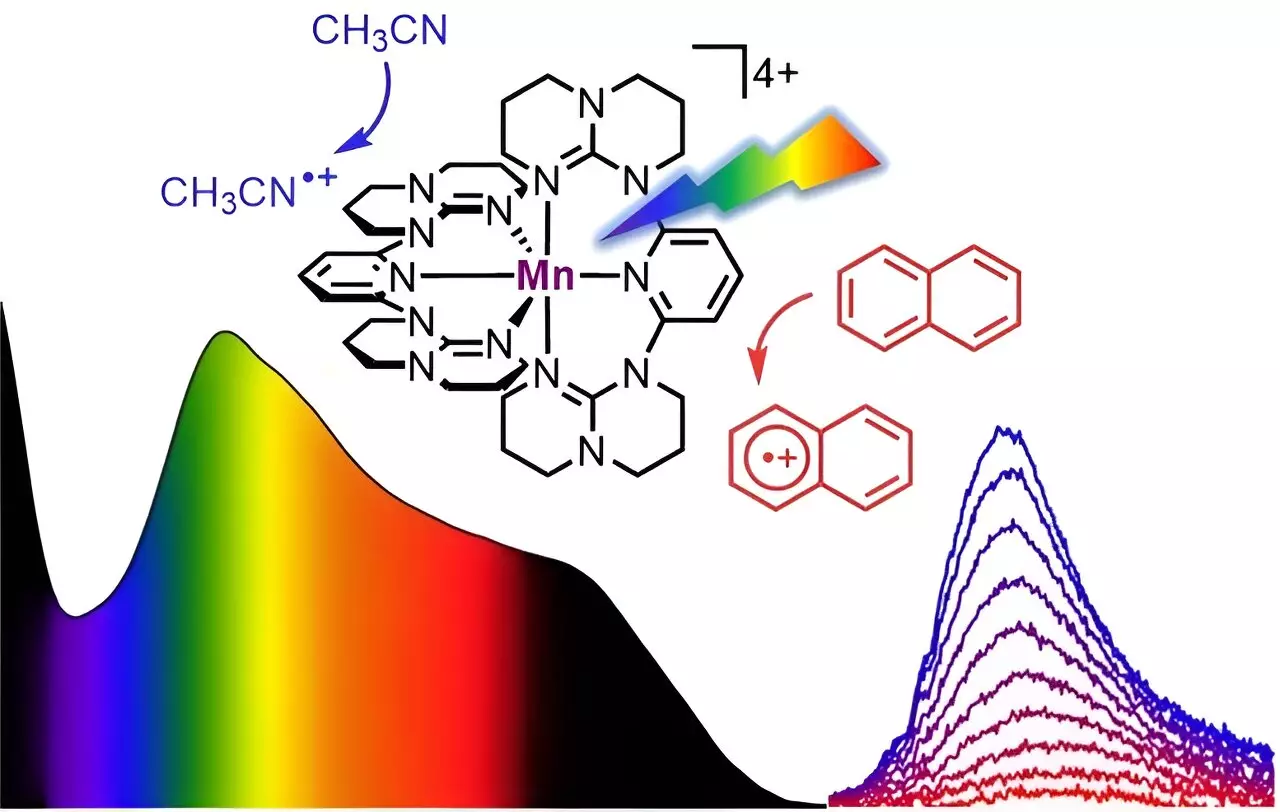
The development of a molecular system based on manganese opens up new avenues for photocatalysis. Compared to precious metals, manganese is the third most abundant metal globally, after iron and titanium, making it a readily available and cost-effective alternative. The soluble manganese complex designed by Professor Katja Heinze’s team displays comprehensive absorption of visible light from blue to red, encompassing a wavelength of 400 to 700 nanometers. Additionally, it can also absorb parts of the near-infrared light up to 850 nanometers. This panchromatic absorption ability is analogous to the dark color of Braunstein, or manganese dioxide, a naturally occurring mineral. However, unlike the mineral Braunstein, the “molecular Braunstein” emits NIR-II light with a wavelength of 1,435 nanometers when excited by visible or NIR-I light with a wavelength of 850 nanometers. This emission in the NIR-II region is unprecedented for a molecular manganese system, even surpassing the performance of noble metals.
The unique observation that the “molecular Braunstein” can oxidize various organic substrates adds another layer of intrigue to this novel manganese-based system. The photocatalyst demonstrated the capability to oxidize highly challenging aromatic molecules with exceptional oxidation potentials, including naphthalene, toluene, and benzene. Moreover, stable solvents were also susceptible to the superphotooxidant when excited by LED light. These findings highlight the promising oxidizing power of the “molecular Braunstein” and its potential to revolutionize light-driven reactions.
Through the use of ultrafast spectroscopic techniques, the research team uncovered two distinctive photoactive states in the molecular manganese system. The first state, characterized by a short-lived but highly oxidizing high-energy state, allows the photocatalyst to attack solvent molecules in close proximity before light excitation. The second state, which persists for a longer duration, possesses moderately oxidizing lower-energy properties, enabling the oxidation of aromatic substrates following diffusional collision. This insight into the excited-state behavior of the “molecular Braunstein” showcased the phenomena of static and dynamic quenching of the excited states.
To gain a comprehensive understanding of the photoinduced processes at play, the research team employed quantum chemical calculations alongside the spectroscopic results. These sophisticated calculations were made feasible by the computing power of the supercomputers MOGON and ELWETRITSCH in Rhineland-Palatinate. Dr. Christoph Förster, a senior scientist, played a vital role in the quantum chemical study, further contributing to the advancement of photochemistry.
The discovery of a highly efficient and cost-effective manganese-based photocatalyst paves the way for the development of novel light-driven reactions. By replacing the rare and expensive ruthenium and iridium compounds that are currently prevalent, scientists can create more sustainable and accessible reactions. Additionally, this breakthrough opens doors to previously unattainable reaction and substrate classes. Professor Katja Heinze and her team, equipped with their own ultrafast laser system, the computing power of high-performance supercomputers, and the skills of their Ph.D. students, are committed to advancing sustainable photochemistry.
The development of a soluble manganese complex with panchromatic absorption and NIR-II emission marks a significant milestone in photocatalysis. Harnessing the power of manganese, an abundant and cost-effective metal, this molecular system demonstrates unparalleled efficiency in oxidizing organic substrates. The discovery contributes to the ongoing efforts to replace precious metals and enhance the sustainability of photochemical reactions. Exciting possibilities lie ahead as scientists continue to explore the potential of this groundbreaking manganese-based photocatalyst.

Leave a Reply