Ammonia is an essential compound used in fertilizers, and the traditional method of production through the Haber-Bosch process involves high pressure and temperatures, leading to a significant energy footprint. However, researchers at the RIKEN Center for Sustainable Resource Science (CSRS) have discovered a greener way to produce ammonia that is not only more eco-friendly but also cost-effective. This breakthrough is a game-changer in the quest for a carbon-neutral and green-energy economy.
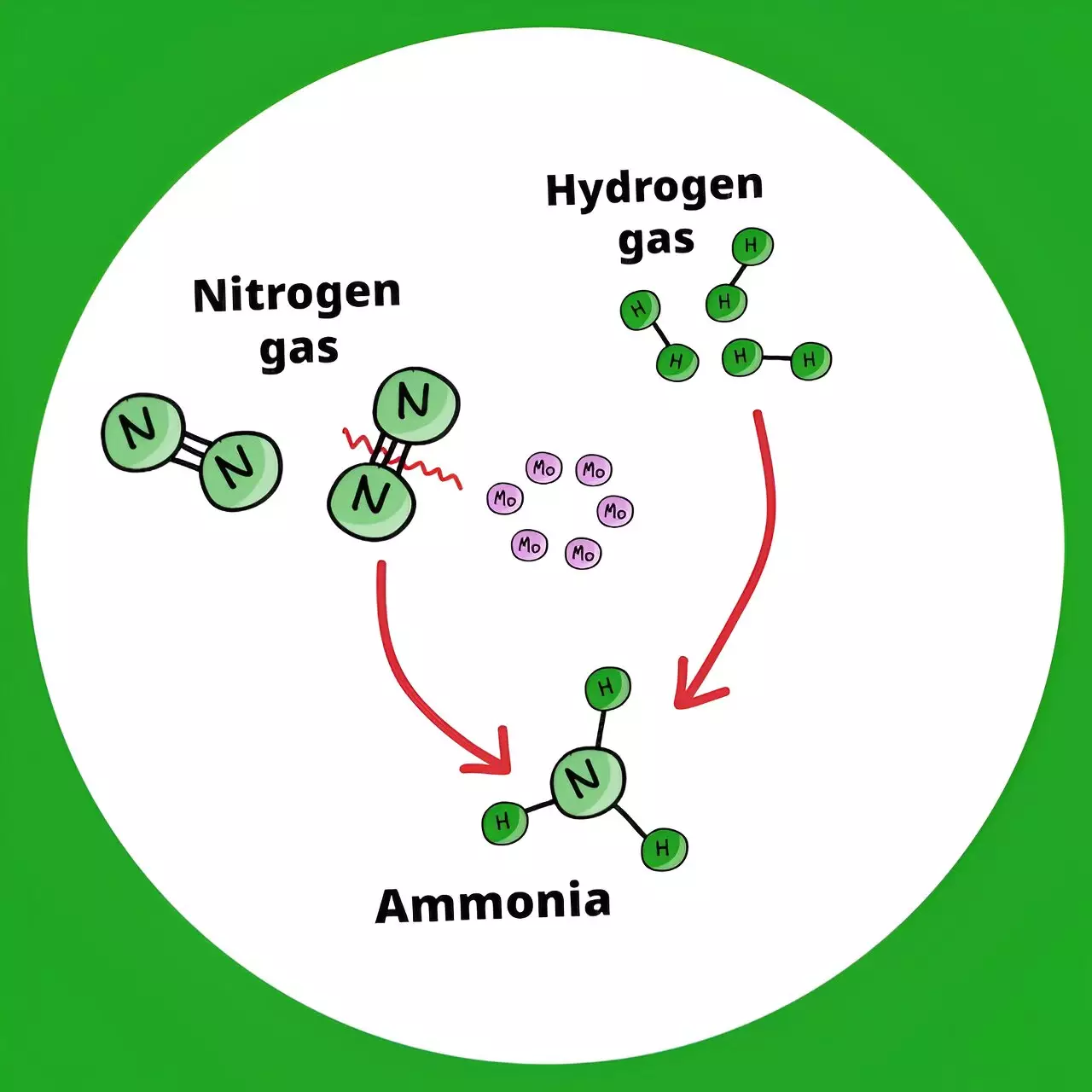
The key to this innovative approach lies in a new catalyst developed by the researchers at RIKEN CSRS. This catalyst operates efficiently at lower temperatures, reducing the energy and costs associated with synthesizing ammonia. By using ultrasmall molybdenum metal particles prepared from a hexanuclear molecular metal halide cluster, activated with hydrogen gas, the catalyst effectively breaks down the strong nitrogen-nitrogen bonds and drives ammonia synthesis rapidly. This breakthrough opens up new possibilities for sustainable ammonia production.
The conventional method of producing ammonia through the Haber-Bosch process consumes a vast amount of energy due to the high pressure and temperatures required. With the new catalyst developed by the RIKEN CSRS researchers, the energy footprint of ammonia production can be significantly reduced. The ability to synthesize ammonia at much lower temperatures without losing efficiency is a major step towards a more sustainable future.
In addition to revolutionizing the fertilizer industry, the new method of producing ammonia has the potential to reduce carbon emissions on a global scale. Ammonia fuel, which can be burned directly in internal combustion engines without emitting CO2, could become a practical alternative with the lower-energy production process. This would not only reduce carbon emissions but also contribute to the prevention of further global warming, making a significant impact on the environment.
While the new catalyst offers a more sustainable approach to ammonia production, challenges remain, particularly concerning the production of hydrogen needed for the synthesis process. Currently, hydrogen production still relies on fossil fuels, leading to significant CO2 emissions. However, by combining the new catalyst system with green hydrogen production from renewable sources, the emission of global-warming CO2 could be further reduced. The researchers are actively working on improving the efficiency of ammonia synthesis by adding promoters to the molybdenum-based catalyst.
The discovery of a greener way to produce ammonia marks a significant milestone in the journey towards a sustainable and carbon-neutral future. By reducing energy consumption, lowering carbon emissions, and offering a more efficient method of ammonia production, this breakthrough has the potential to reshape the way we approach energy production and environmental sustainability. With further research and development, the impact of this innovation could be felt on a global scale, paving the way for a cleaner and more sustainable future for generations to come.

Leave a Reply