The quest for sustainable water disinfection has led researchers at the University of Pittsburgh, Drexel University, and Brookhaven National Laboratory to explore the possibilities of electrochemical ozone production (EOP) technologies. These technologies have the potential to revolutionize water disinfection by offering a more sustainable alternative to centralized chlorine treatments. Unlike chlorine, ozone generated through EOP naturally decomposes in water, making it a safer option for drinking water, swimming pools, and hospital use.
While the promise of EOP is great, the practical challenges in developing efficient, economical, and sustainable EOP technologies are significant. One of the key obstacles is the lack of understanding at the molecular level of how EOP works and what makes a good catalyst for the process. Without a reliable catalyst, EOP remains too expensive and energy-intensive for widespread use.
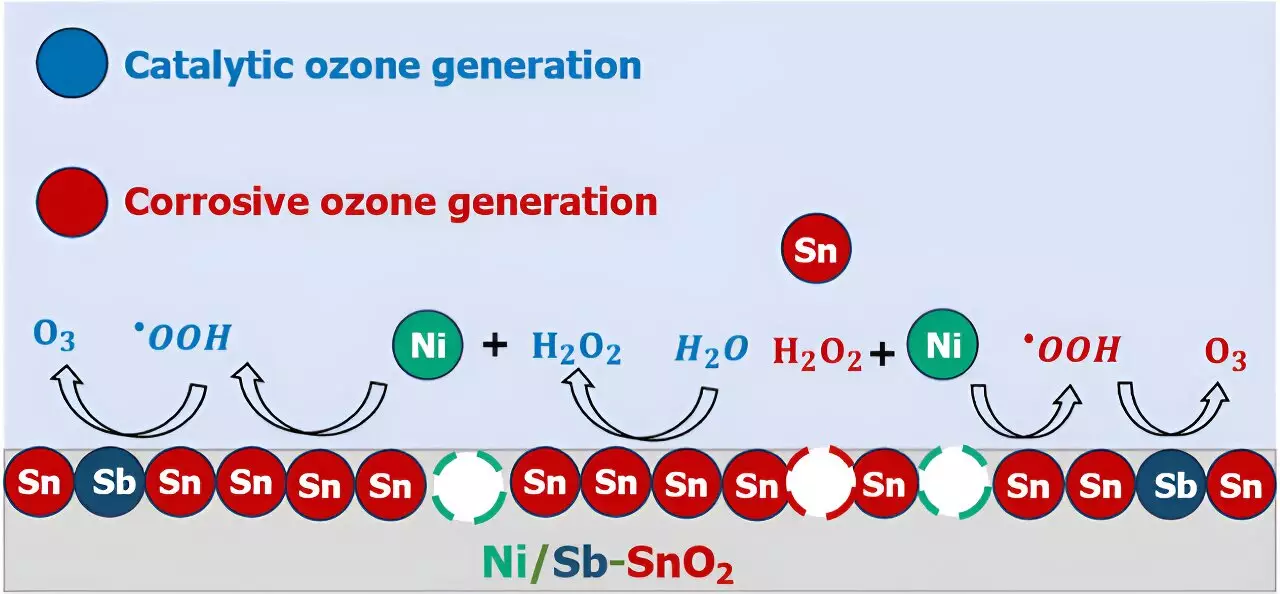
To address this challenge, researchers are working to decode the mysteries of EOP catalysts, particularly focusing on nickel- and antimony-doped tin oxide (Ni/Sb–SnO2, or NATO), one of the most promising EOP catalysts known to date. By using experimental electrochemical analyses, mass spectrometry, and computational quantum chemistry modeling, researchers have been able to uncover the atomic-scale processes that lead to ozone generation on NATO electrocatalysts.
One of the surprising findings of the research is the role of corrosion in the EOP process. It was discovered that some of the nickel in NATO catalysts leaches out of the electrodes via corrosion and floats in the solution where it can promote chemical reactions that lead to ozone generation. This dual mechanism involving both catalytic ozone formation and catalyst decomposition poses a challenge in developing stable and efficient EOP catalysts.
Understanding the complex interplay between catalyst corrosion, solution phase reactions, and ozone generation is crucial for improving EOP technologies. By identifying the fundamental constraints and mechanisms at play in EOP, researchers can pave the way for the development of better catalysts that are more resistant to corrosion and promote economically and sustainably viable EOP.
The journey towards sustainable water disinfection through electrochemical ozone production is a challenging one, but the insights gained from decoding EOP catalysts are a step in the right direction. As researchers continue to uncover the atomic-level processes that govern EOP, the possibility of scaling up this technology to a global level becomes a more achievable goal. By finding new atomic combinations in materials and addressing the challenges of corrosion and solution phase reactions, the dream of sustainable water disinfection through EOP may soon become a reality.

Leave a Reply