Somatostatin receptors (SSTRs) are a crucial family of receptors that play a vital role in regulating hormone secretion and inhibiting tumor growth. Among the five subtypes, SSTR5 is highly expressed in the pituitary gland and controls the release of important hormones.
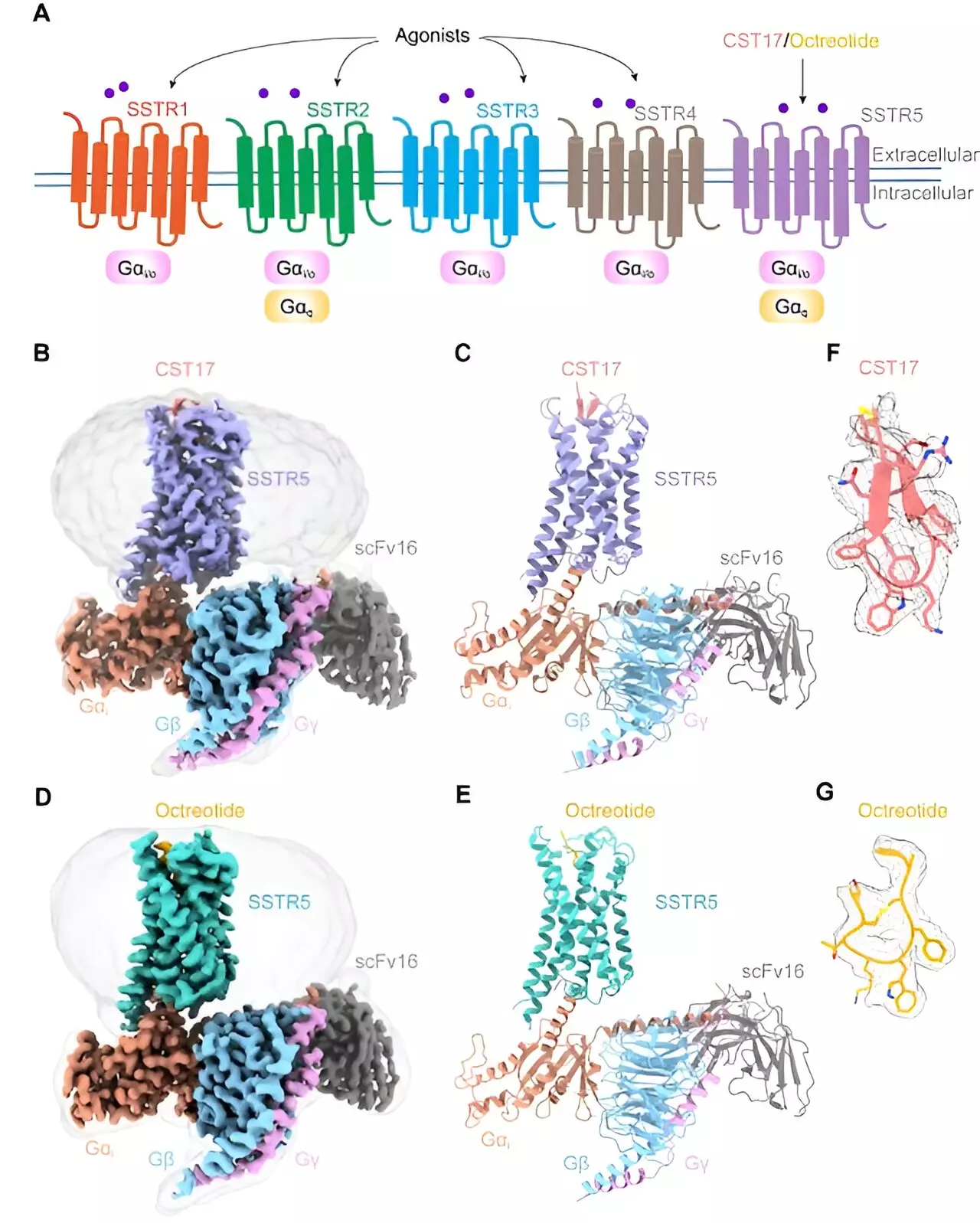
A study published in PNAS by a research team from the Shanghai Institute of Materia Medica utilized single-particle cryo-electron microscopy techniques to uncover the three-dimensional structures of SSTR5 in complex with agonists CST17 and octreotide. The detailed analysis provided insights into the molecular mechanisms underlying SSTR5 activation by these ligands.
Structural Revelations and Functional Implications
The cryo-EM structures revealed that the binding of agonists like CST17 and octreotide triggered a conformational change in SSTR5, leading to the activation of G proteins and subsequent signaling cascades. Moreover, the study uncovered distinct recognition modes of extracellular loops for the two agonists, shedding light on their selectivity and specificity.
Understanding the activation mechanisms of SSTR5 opens up new avenues for the design of highly selective modulators with reduced off-target effects. This has significant therapeutic implications for various conditions such as acromegaly, pituitary adenomas, neuroendocrine tumors, and hormonal imbalances.
The study provides valuable insights into the structural basis of SSTR5 activation and its selective recognition of neuropeptide and drug agonists. This knowledge lays the foundation for the development of novel therapeutic interventions targeting SSTR5, offering hope for improved treatments for a wide range of endocrine disorders and tumors.

Leave a Reply