Chemists at Yokohama National University have made a significant breakthrough in the field of catalysis, paving the way for a more sustainable approach to chemical synthesis. The development of innovative catalysts containing a combination of noble metals has shown remarkable efficiency in ester-producing chemical reactions, all while utilizing oxygen as the sole oxidant. This marks a crucial step towards greener chemistry practices and a more environmentally friendly future.
Cross-Dehydrogenative Coupling (CDC) reactions play a vital role in organic synthesis and industrial chemistry. These reactions involve the activation of C-H bonds, a key process in forming chemical compounds. The CDC reactions between arenes and carboxylic acids are particularly significant as they directly produce aryl esters, which are widely used in industries like pharmaceuticals and polymer production. However, traditional methods using hazardous oxidants have raised environmental and safety concerns.
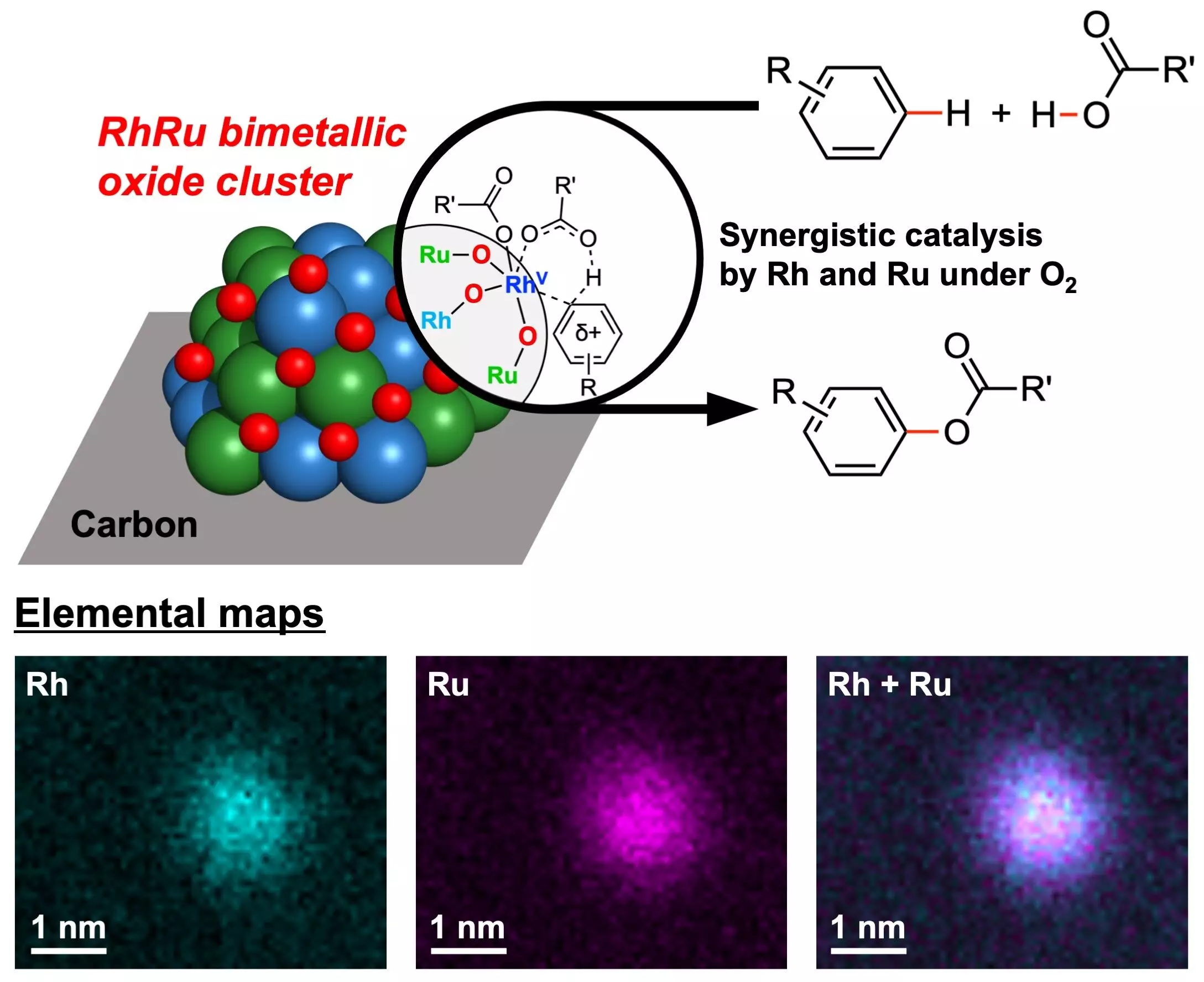
In response to the need for greener catalysts, the research team at Yokohama National University developed RhRu bimetallic oxide clusters (RhRuOx/C). These catalysts, composed of the noble metals Rhodium (Rh) and Ruthenium (Ru), demonstrated exceptional catalytic activity in CDC reactions while utilizing oxygen as the sole oxidant. With advanced imaging and spectroscopic techniques, the researchers confirmed the structure and formation of these clusters, highlighting their potential for sustainable chemical synthesis.
The use of molecular oxygen as the sole oxidant in these CDC reactions is a significant advancement towards greener chemistry practices. Unlike traditional oxidants, molecular oxygen is non-toxic, abundant, and environmentally benign. It efficiently converts reactants to products, with water being the only byproduct. The high reactivity of the RhRu bimetallic oxide clusters with various types of arenes and carboxylic acids makes them versatile for producing aryl esters, showcasing their potential for driving sustainable chemical synthesis.
The development of noble-metal-based bimetallic oxide clusters opens up new possibilities for more sustainable and efficient chemical reactions. The researchers at Yokohama National University plan to further explore the use of these catalysts in other important reactions, with the goal of establishing regioselective C-H functionalization reactions under mild conditions. This represents a significant step towards promoting environmentally friendly chemistry practices and creating a more sustainable future for chemical synthesis.

Leave a Reply