The quest for efficient and sustainable chemical synthesis continues to drive innovation within the fields of chemistry and pharmacology. A noteworthy advancement comes from researchers at the University of Illinois Urbana-Champaign, who have developed a novel catalyst designed to streamline the synthesis of ethers. Ethers are essential chemical compounds found in numerous products, including pharmaceuticals, personal care items, and food. This innovative approach, led by Professor M. Christina White, has been detailed in a recent publication in *Science*, highlighting both its practicality and its unique inspirations rooted in natural processes.
At the core of this research is an exploration of the limitations inherent in conventional ether synthesis methods. Typically, synthesizing ethers involves combining an alcohol with an alkene—a task that, when done using standard laboratory protocols, often leads to a cocktail of various products. This traditional process requires the activation of the alcohol through a proton removal, which not only complicates the desired outcome but also demands sizable quantities of reactants. The inefficiency of generating sufficient amounts of any specific ether from a complex mixture underscores the need for advancements in this field.
Graduate student Sven Kaster, the lead author of the study, expressed frustration with these limitations, stating, “We did not want to activate the alcohol, and we didn’t want to have to use large quantities of the reaction partners.” The realization that an alternative approach might pave the way to more efficient synthesis prompted the research team to seek inspiration from the world of biology, specifically the efficient reactions catalyzed by enzymes.
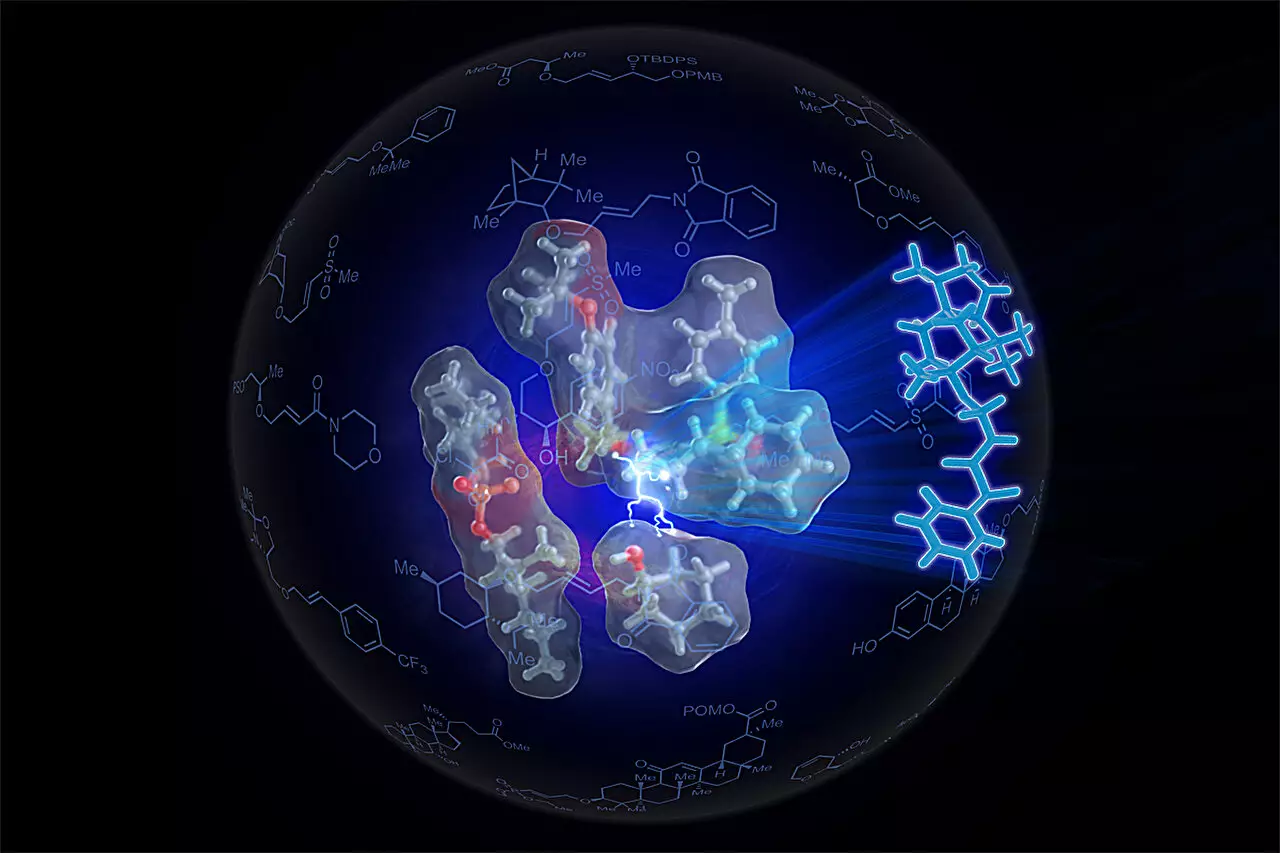
The researchers turned to the concept of self-assembling small-molecule catalysts that mimic enzyme behavior. They incorporated palladium, a metal known for its catalytic properties, into a newly designed catalyst they termed SOX. However, the creation of such a catalyst was only the first step. To truly optimize ether synthesis, alignment and proximity—key characteristics of enzymatic reactions—needed to be systematically engineered into the catalyst’s design.
Professor White adeptly elaborates on this challenge: “It’s like if two people want to hold hands, they have to be close together. But to do it comfortably, they also have to be facing the right way.” This analogy aptly captures the essence of their innovation—the researchers engineered a variant of the SOX catalyst, called Sven-SOX, that not only activated the alkenes but also ensured that they were properly aligned with the alcohol for a productive reaction.
Unlocking New Possibilities in Ether Production
The implications of the Sven-SOX catalyst extend far beyond mere efficiency. The researchers successfully synthesized over 130 different ethers, many of which were previously challenging to create with existing methodologies. “The main advantage to our approach is the generality,” states Kaster, highlighting the versatility of the new technique. The capability to produce complex ethers that would ideally require more intricate and resource-intensive methods opens new avenues for both scientific research and practical applications.
In addition to being versatile, the synthesis process using the Sven-SOX catalyst operates under mild conditions. This is particularly important as it allows for the incorporation of sensitive functional groups that would typically be compromised under harsher traditional methods. Moreover, the efficiency of the procedure is such that even middle school students could theoretically undertake it, reflecting a landmark simplification in chemical process design.
With the successful demonstration of the Sven-SOX catalyst, the research team is now poised to target other classes of chemicals that could benefit from enzyme-like small-molecule catalysts. The possibilities for further application of their findings are considerable, as they seek to refine the properties of these catalysts and experiment with their performance in additional chemical reactions.
Professor White gives credence to the significant potential this research holds, underscoring the importance of basic scientific inquiry in uncovering the vast capabilities of small molecules that can imitate enzymes. The journey toward optimizing ether reactions represents just the beginning of a broader agenda aimed at transforming how chemists approach synthetic challenges.
In summation, the efforts at the University of Illinois Urbana-Champaign not only contribute to the rich landscape of chemical synthesis but also accentuate the invaluable connection between nature and scientific innovation. The work is a testament to the power of interdisciplinary thinking—that by looking to the natural world, researchers are finding pathways to advance synthetic methodologies, with implications that resonate throughout various sectors of industry and research.

Leave a Reply