As the world grapples with the growing threat of climate change, the search for effective strategies to repurpose carbon dioxide (CO2) has become a critical undertaking. A recent study published in *Nature Energy* endeavors to illuminate the complex pathways through which CO2 can be transformed into valuable chemicals such as ethylene and ethanol. This research promises profound implications for industries reliant on chemical processes, combining advanced experimental techniques with theoretical models to forge a path towards sustainability in chemical manufacturing.
The study, spearheaded by an accomplished team including Dr. Arno Bergmann and Professors Beatriz Roldán Cuenya and Núria López, utilized cutting-edge technologies such as in-situ surface-enhanced Raman spectroscopy (SERS) and density functional theory (DFT). These methods facilitated a nuanced examination of the molecular species on copper (Cu) electrocatalysts, crucial for the electrochemical reduction of CO2 (CO2RR). The research zeroes in on understanding how specific catalysts operate during the conversion process, shedding light on previously obscure mechanisms and providing a crucial foundation for the design of more effective catalysts.
The ultimate goals of this research extend beyond theoretical knowledge; they aim to enable the chemical industry to utilize CO2 not only effectively but sustainably. By focusing on the production of ethylene and ethanol—key building blocks for environmentally-friendly plastics and fuels—the study taps into a dual benefit: contributing to green chemistry while addressing CO2 emissions.
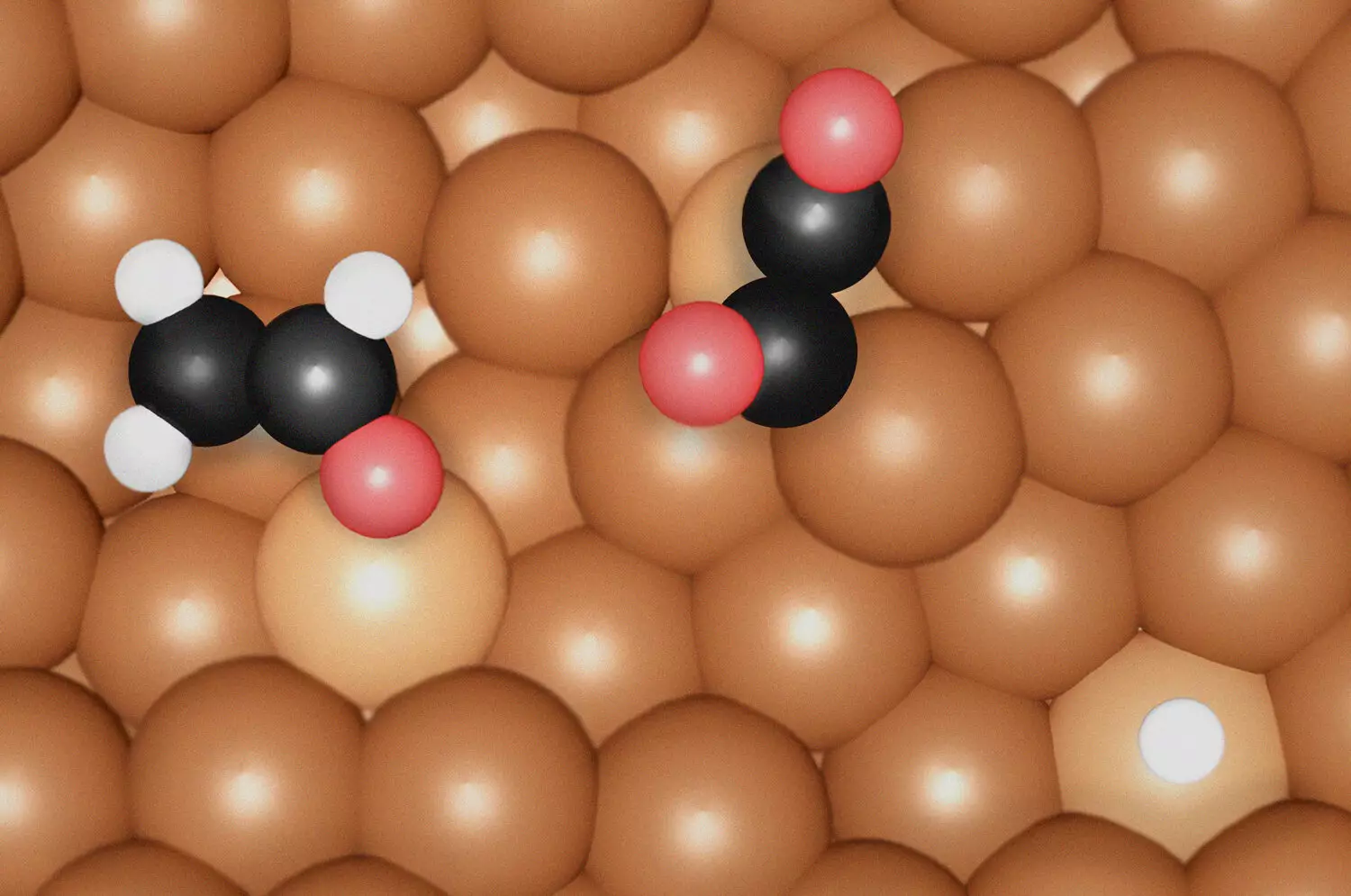
In their investigation, the research team identified distinct intermediates that play pivotal roles in the production of ethylene and ethanol. The formation of ethylene occurs through the assembly of specific intermediates known as *OC-CO(H) dimers on undercoordinated Cu sites. These dimers are integral to the pathway that leads to ethylene synthesis. On the other hand, ethanol production necessitates a unique environment characterized by a compressed and distorted coordination of Cu sites, with the key intermediate identified as *OCHCH2. This differentiation is crucial, as it reveals the tailored requirements for synthesizing each chemical, allowing for targeted catalyst design.
Moreover, the research emphasized the significance of surface morphology in determining catalyst efficiency. The undercoordinated Cu sites found under reaction conditions amplify the binding strength of carbon monoxide (CO), a vital precursor in the CO2 reduction reaction. The study highlights that these irregularities at the atomic level not only influence the reaction’s progress but are adaptable based on reaction conditions, further enhancing the efficacy of the catalytic surface.
The insights gleaned from this study hold transformative potential for the chemical industry. By comprehending the specific conditions necessary for the selective production of ethylene and ethanol, researchers can innovate the design of catalysts that are not only more efficient but also significantly more sustainable. With the ability to utilize CO2 effectively, industries can markedly reduce their carbon footprints, aligning with global sustainability goals.
The collaboration between interdisciplinary research groups, specifically between teams in Germany and Spain, illustrates the value of combining theoretical perspectives with empirical data. This synergetic approach amplifies the understanding of CO2 reduction processes, providing context and substantiation for enhanced catalytic designs.
The groundbreaking work conducted by the research team at the Interface Science Department of the Fritz Haber Institute and the Institute of Chemical Research of Catalonia offers a monumental leap forward in the realm of CO2 reduction. By identifying key intermediates and active sites for the effective production of ethylene and ethanol, this research lays the groundwork for developing sustainable catalytic processes. The implications of such advancements reach far beyond academic interest; they propose practical solutions to mitigating CO2 emissions while fostering sustainable practices across the chemical industry. As we advance towards a more sustainable future, this study stands as a testament to the potential of innovative research and collaboration in addressing one of the most pressing challenges of our time.

Leave a Reply