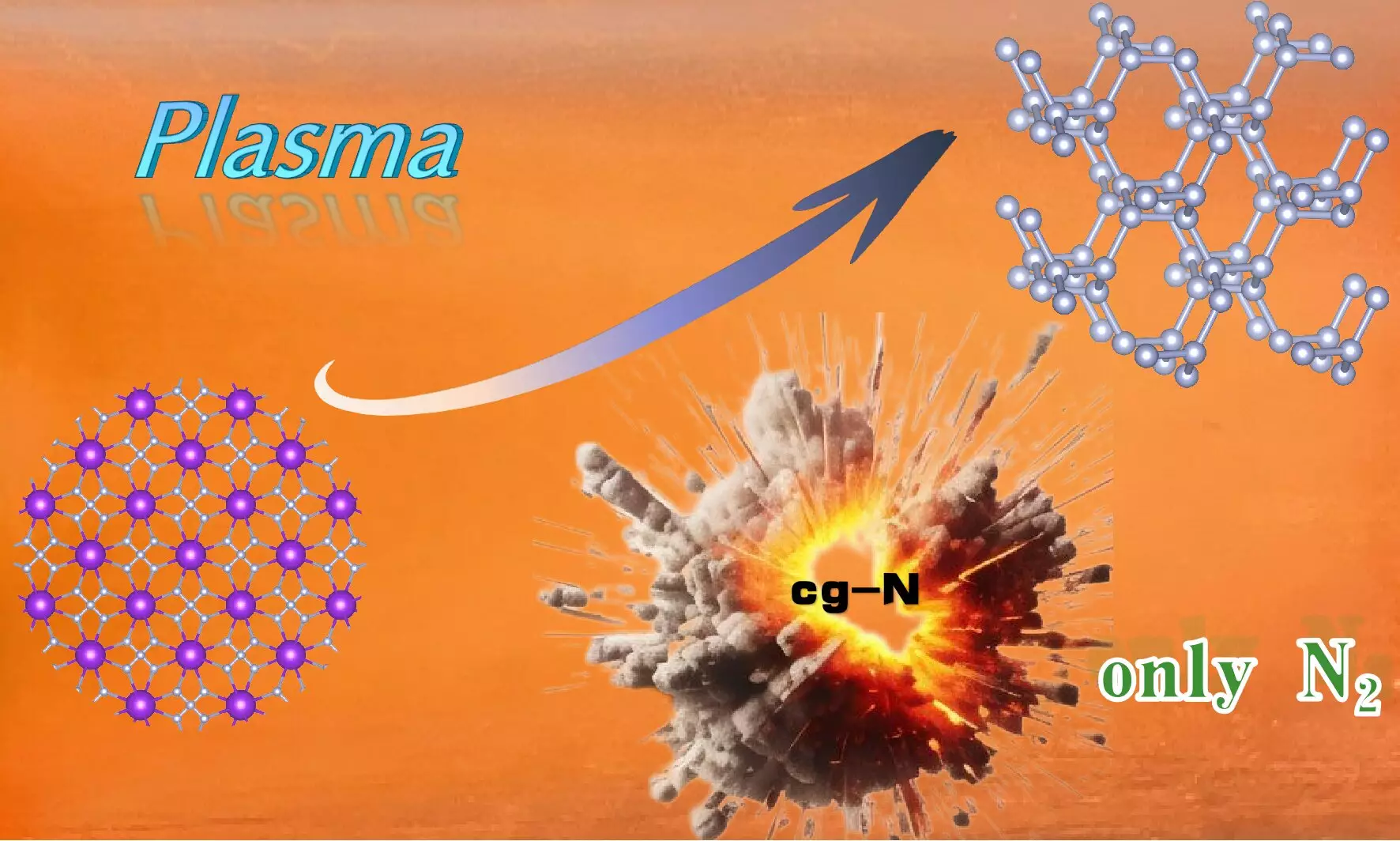
In the realm of materials science, the pursuit of high-energy-density materials has gained significant traction, primarily due to their potential applications in energy storage and propulsion systems. A groundbreaking development has emerged from the research spearheaded by Professor Wang Xianlong and his team at the Hefei Institutes of Physical Science, under the auspices of the Chinese Academy of Sciences. Their recent publication in *Science Advances* details the synthesis of cubic gauche nitrogen (cg-N), a novel nitrogen allotrope notable for its unique structure akin to diamond.
The team’s approach to synthesizing cg-N involved the utilization of plasma-enhanced chemical vapor deposition (PECVD) on potassium azide (KN3) as a precursor substance. The decision to use KN3 is particularly significant, not only because of its lower toxicity and risk of explosion compared to alternative precursors, but also due to the strong electron transfer capacity that potassium provides. This innovative method allows for the production of cg-N at atmospheric pressure, an achievement that addresses the existing challenges linked to synthesizing high-energy-density materials safely and efficiently under normal atmospheric conditions.
Fundamental to the successful synthesis of cg-N was the comprehensive theoretical framework established by the research team. They employed first-principles calculations to analyze the structural stability of cg-N surfaces across varying conditions—specifically, changes in pressure and temperature. Their findings underscored a crucial insight: surface instability was a primary factor leading to the decomposition of cg-N at lower pressures. This revelation not only enhanced our understanding of cg-N’s physical characteristics but also facilitated the proposal of methods to stabilize it, such as saturating surface bonds and enhancing charge transfer, extending cg-N’s thermal stability up to about 750 K.
The thermal behavior of the synthesized cubic gauche nitrogen was rigorously evaluated through thermogravimetric-differential scanning calorimetry (TG-DSC). The results were promising, revealing that cg-N possesses significant thermal stability up to 760 K before undergoing rapid thermal decomposition. This characteristic is pivotal, as it signifies potential applications in environments where thermal stability is paramount, further propelling cg-N into the spotlight amongst energy materials.
The implications of this research transcend academic curiosity; they herald a new era in the development of high-energy-density materials. With the demonstrated ability to synthesize cg-N at atmospheric pressure efficiently, the team’s innovative approach opens avenues for exploring the full potential of nitrogen-based materials in various applications, including batteries, explosives, and propulsion systems. This groundbreaking work not only sets the stage for future explorations into other high-energy-density compounds but also serves as a catalyst for advancing our understanding and practical capabilities in materials engineering.
The emergence of cubic gauche nitrogen, synthesized through the innovative efforts of Professor Wang Xianlong’s team, represents a significant leap forward in both theoretical and practical aspects of high-energy-density materials. As the field evolves, continues research and development may pave the way for novel applications that could revolutionize energy storage and related technologies.

Leave a Reply