In the quest for sustainable energy solutions, the reduction of carbon dioxide (CO₂) presents both a challenge and an opportunity. Recent research conducted by a team led by Georgios Katsoukis at the University of Twente signifies a pivotal shift in understanding how chemical environments can significantly alter the efficiency of CO₂ conversion processes. This study underscores that while catalyst materials have long been the center of attention, the surrounding chemical environment, particularly in electrochemical reactions, plays a crucial role as well.
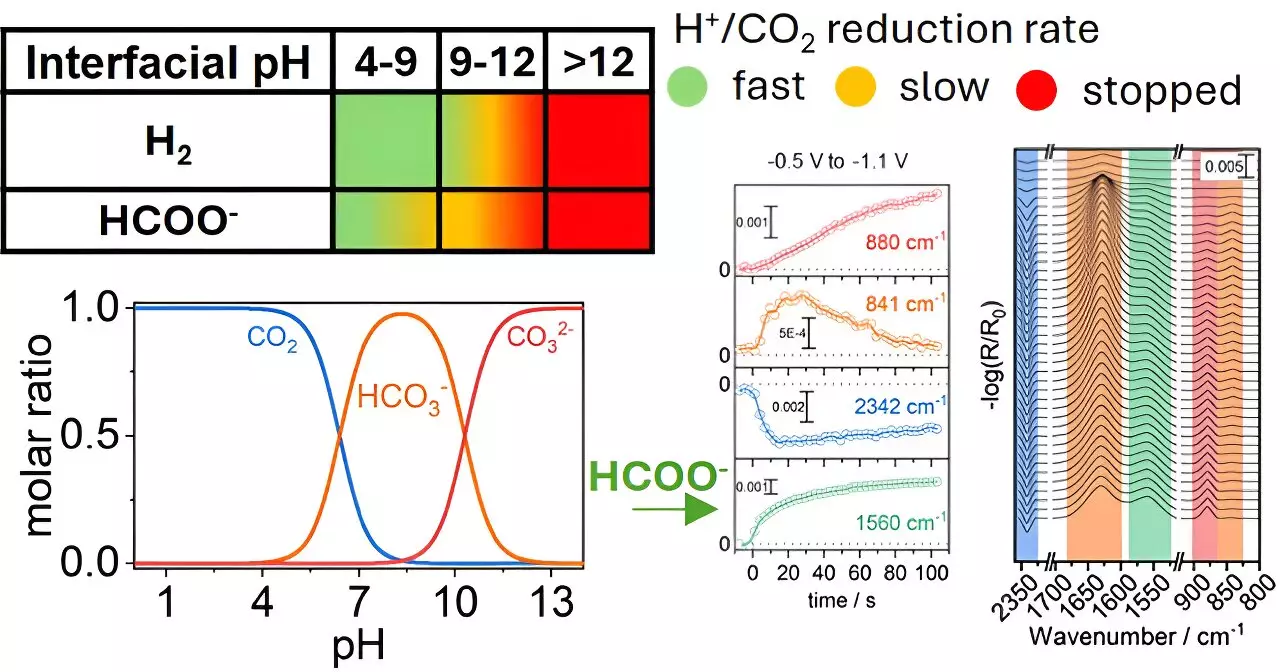
The key revelation from Katsoukis and his colleagues is the impact of pH levels in the vicinity of copper electrodes during CO₂ reduction. Their data suggests that manipulating these local chemical conditions can not only enhance the selectivity of the reaction but also improve the overall efficiency of the conversion process. This finding opens the door for innovative strategies in experimental designs, positioning the chemical environment as an equal partner in catalyst development rather than a mere backdrop.
The conversion of CO₂ into formate—a critical intermediate with vast industrial applications—demonstrates the practical significance of this research. Formate is not merely an end product; it can serve as a precursor in various chemical processes, including the synthesis of fuels and chemicals, potentially creating a closed-loop system that benefits the economy and the environment alike.
However, generating formate is not straightforward; a multitude of byproducts can arise due to varying reaction conditions. This issue has posed a longstanding challenge in the field of CO₂ reduction. The research findings suggest that by fine-tuning the local chemical settings around the copper electrodes, researchers can selectively steer the reactions toward more desirable outcomes while minimizing unwanted byproducts. This nuanced approach could lead to more predictable and controllable industrial processes.
The implications of this research extend beyond immediate chemical conversions. It serves as a blueprint for advancing CO₂ reduction technologies in a way that prioritizes a holistic understanding of the reaction environment. As scientists delve deeper into the interactions between catalysts and their surroundings, there’s the potential for developing customized electrochemical systems that are both more efficient and longer-lasting.
By integrating chemical environment modifications into the catalyst optimization process, it is conceivable to create electrochemical systems that significantly reduce CO₂ emissions while transforming them into economically valuable products. This integrated strategy could help pave the way toward a sustainable and circular economy, where carbon emissions are viewed not as waste but as a resource waiting to be harnessed.
Ultimately, the findings from Katsoukis and his team represent a significant leap in the research on CO₂ reduction. By addressing the multifaceted nature of these reactions—including the importance of local pH and electrochemical conditions—the team highlights a necessary evolution in the way researchers approach catalyst design. With a clear pathway for future investigations and an emphasis on optimizing both materials and their environments, we come closer to realizing practical applications that could transform the reduction of CO₂ into a cornerstone of sustainable industry.

Leave a Reply