In a pioneering advancement, researchers at Oak Ridge National Laboratory (ORNL) have successfully merged two analytical techniques to simultaneously detect fluorine and various isotopes of uranium within the same particle. This dual-detection capability represents a significant leap forward in the analysis of nuclear materials, offering potentially critical insights for the International Atomic Energy Agency (IAEA) in determining the intended use of these materials. The implications of this research extend beyond nuclear nonproliferation; it opens new avenues for applications in other scientific fields, including environmental science and advanced material manufacturing.
Fluorine plays a vital role in the process of uranium enrichment, which is critical for both civilian nuclear power generation and potential weapons development. The ability to identify fluorine in conjunction with uranium isotopes allows for a nuanced understanding of the processes that produce nuclear materials. By determining the ratios of these elements, inspectors can infer the provenance of a sample, as well as the specific methods employed to create it. This is particularly important in the context of national security, where trace elements can unveil information about a sample’s history and experiments that may have occurred.
Rapid Characterization of Single Particles
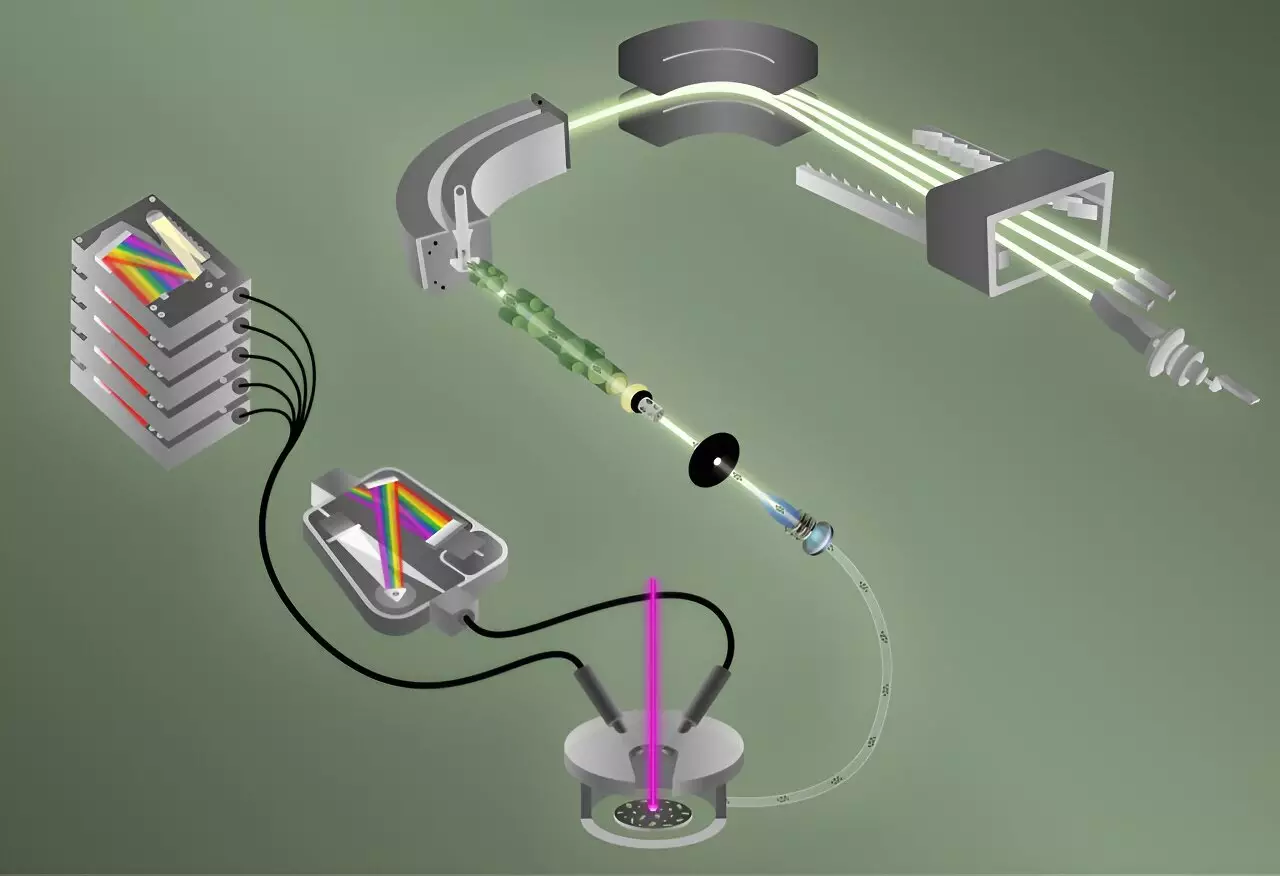
Benjamin Manard, the lead scientist of the study, emphasizes that traditional methods of determining isotopic ratios in single particles are often time-consuming. ORNL’s innovative approach enables rapid analysis of 40 individual particles—each comparable in size to a red blood cell—within a mere five minutes. This efficiency is attributed to the integration of two cutting-edge techniques: laser-induced breakdown spectroscopy (LIBS) and laser ablation multicollector inductively coupled plasma mass spectrometry (ICP-MS).
LIBS is celebrated for its ability to quickly identify elements thanks to its sensitivity. The process involves vaporizing a sample, which generates a plasma comprising excited ions that emit light as they cool. The unique wavelengths of this emitted light correspond to specific elements, revealing the presence of fluorine in the sample. Manard likens this to a fireworks display, where different colors signify different elements.
In a simultaneous process, helium gas aids in transferring the vaporized particles into a mass spectrometer utilizing ICP-MS. This secondary technique specializes in analyzing isotopes of uranium, uncovering their specific ratios within the sample. The use of a mass spectrometer requires positive ions, a feature readily exhibited by many metals, including uranium, but not by fluorine which prefers a negative charge. The research team’s innovative combination of these techniques provides a comprehensive analysis, marking the first-time integrated measurement of both fluorine and uranium isotopes.
Implications for Nuclear Nonproliferation
The dual detection method has substantial implications for nuclear nonproliferation. Brian Ticknor, an ORNL co-author, highlights the importance of understanding the fluorine to uranium ratio within samples. Insights into how and when those materials were produced can shed light on nuclear activities and intentions. This capability serves as an invaluable tool for international regulatory bodies that monitor countries’ adherence to nuclear agreements.
Moreover, while the primary focus of this research is on nuclear materials, the techniques hold promise for applications in diverse fields. The analysis methods could contribute significantly to next-generation battery technologies, fuel advancements for nuclear reactors, and even environmental studies looking at microplastic movements throughout ecosystems.
Future Directions and Broader Applications
The ORNL team envisions extending their techniques to identify other complex compounds relevant to nuclear processes, particularly those containing chlorine, another electronegative element akin to fluorine. The goal is not limited to uranium compounds; they aim to explore various materials involved in nuclear science and technology. This forward-looking approach exemplifies the laboratory’s commitment to pushing the boundaries of particle analysis.
As the scientists refine their methods, they are also investigating the potential for higher throughput analysis, with aspirations to increase the number of particles characterized within a 24-hour period significantly. The team’s previous successes include differentiating between uranium oxide and uranyl fluoride, showcasing the reliability of their methods.
The convergence of LIBS and ICP-MS at Oak Ridge National Laboratory stands to transform the landscape of nuclear material analysis, aligning scientific advancement closely with national security interests. The ability to rapidly and accurately determine the presence and ratios of critical elements not only enhances our understanding of nuclear processes but also strengthens the framework for safety and regulatory compliance in the global landscape of nuclear stewardship. As research continues, the impact of this breakthrough could ripple through various fields, unearthing new pathways for exploration and insight.

Leave a Reply