In the pursuit of a sustainable energy future, hydrogen gas emerges as a key player due to its high energy density and environmentally benign profile. As the lightest and most abundant element in the universe, hydrogen holds immense potential as a clean energy carrier; however, its natural occurrence is primarily in bond form with other elements, which poses challenges for exploitation. The most common hydrogen carriers include ammonia—often overlooked in discussions of hydrogen fuel—thanks to its favorable properties. With hydrogen constituting approximately 17.6% of ammonia’s mass, it not only offers a viable hydrogen storage solution but also benefits from established infrastructure for transportation and storage. This makes ammonia a compelling candidate in the quest for effective hydrogen utilization. Yet, despite its advantages, ammonia’s potential as an on-demand hydrogen source is curtailed by significant technical hurdles.
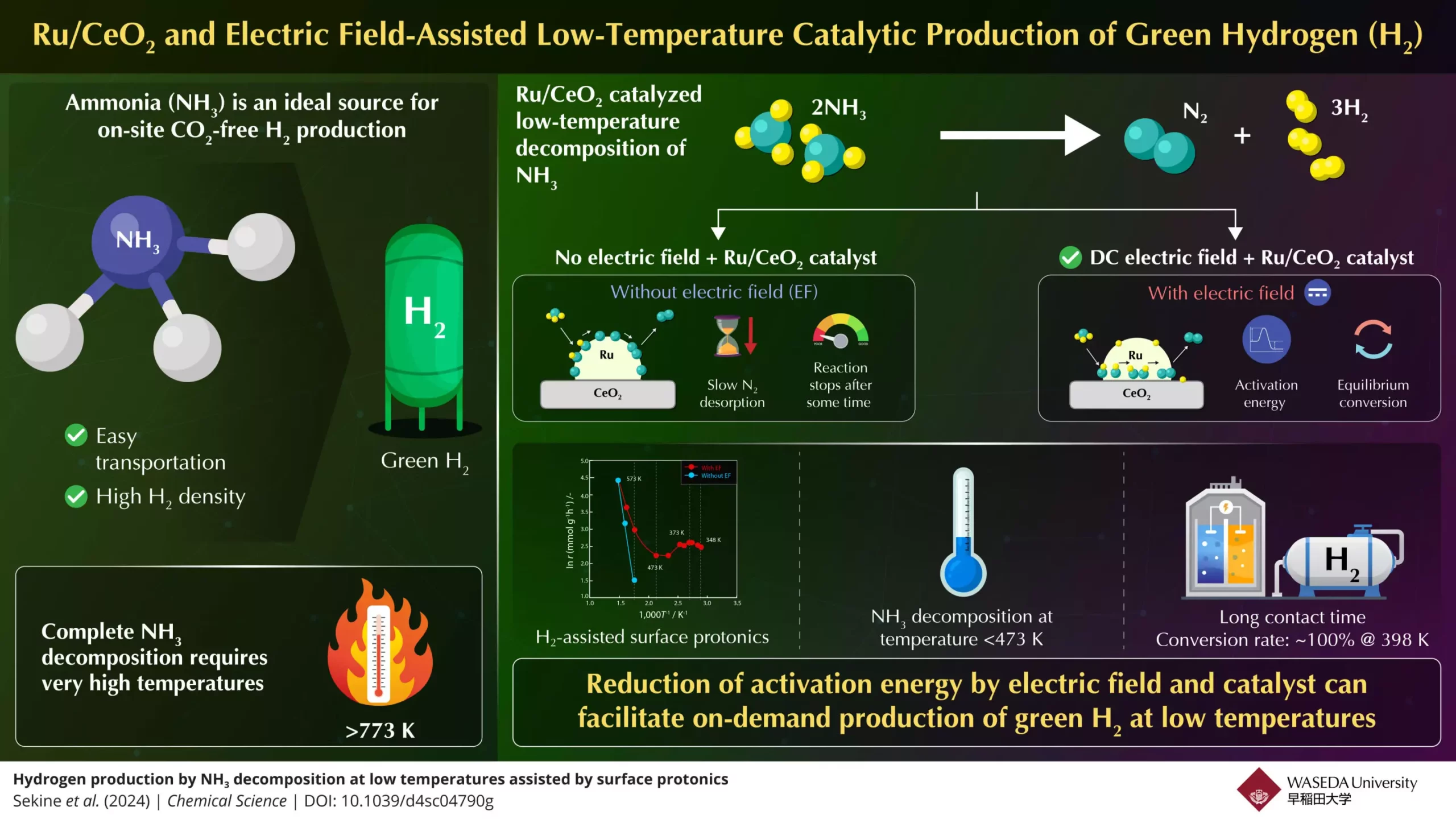
The primary hurdle in leveraging ammonia for hydrogen production is its decomposition process. Current thermal catalytic methods necessitate extremely high temperatures—above 773K—for ammonia to break down into viable hydrogen and nitrogen gas. Such requirements not only limit the scenarios in which ammonia can be efficiently deployed but also render hydrogen production economically challenging. The drive for cleaner and more effective alternatives has intensified research efforts aimed at achieving efficient ammonia conversion at lower temperatures. The inherent complexities associated with nitrogen bond dissociation—part of ammonia’s molecular structure—complicate matters further, making it imperative to find innovative solutions that can circumvent these temperature restrictions.
In a significant breakthrough, a research team led by Professor Yasushi Sekine from Waseda University has presented a promising solution. Collaborating with experts from Yanmar Holdings, this innovative group has developed a compact process that enables ammonia conversion at significantly lower operating temperatures, making it a game-changer in this field. Their methodology involves the utilization of a Ru/CeO2 catalyst in combination with an electric field to enhance the rate of ammonia dissociation. This approach allows the reaction to take place much more efficiently than traditional thermal methods, setting the stage for wider adoption of hydrogen fuel technologies.
The team’s experimental setup demonstrated groundbreaking results by achieving 100% ammonia conversion at a notably low temperature of 398K. This remarkable efficiency surpasses the equilibrium conversion rates typically observed at high temperatures, underscoring the method’s effectiveness. By strategically applying an electric field, the researchers enhanced surface proton conduction on the catalyst, lowering activation energy requirements and streamlining the reaction process.
Central to the improvements realized by Sekine and his team is the incorporation of surface protonics—a process involving proton hopping—as facilitated by the applied electric field. This technique not only accelerates ammonia conversion but also addresses the slow nitrogen desorption process that typically plagues traditional catalytic methods. Without the electric field, the reaction rate would diminish significantly, highlighting the electric field’s crucial role in maintaining a vigorous catalytic activity.
The research team’s findings were further substantiated by a thorough analysis that combined experimental observations with density functional theory calculations. These calculations provided deeper insights into how electric fields could optimally manipulate the catalytic process, representing a significant advance in understanding catalytic efficiency in ammonia decomposition.
This innovative method represents a pivotal step toward the effective integration of ammonia as a hydrogen carrier, facilitating the on-demand synthesis of sustainable hydrogen fuels at lower costs and operational necessities. By making hydrogen production accessible through simple and efficient techniques, this research may significantly advance the transition to clean alternatives in the energy sector.
As energy demands surge and the need for CO2-free energy alternatives escalates, the work led by Sekine and his collaborators stands to pave the way for broader adoption of hydrogen technologies. Their contributions not only address critical challenges in hydrogen production but also offer a blueprint for future developments in energy solutions that prioritize sustainability. Conclusively, the ongoing research highlights the promising role of impactful scientific collaborations in achieving revolutionary advancements in renewable energy technologies.

Leave a Reply