In recent years, the conversation surrounding climate change has largely revolved around the rising levels of carbon dioxide (CO2) in our atmosphere. While the broad implications for weather patterns and global temperatures are well-documented, emerging research indicates that elevated CO2 levels can also profoundly influence cellular mechanics. One significant insight is the interaction of CO2 with hydrogen peroxide (H2O2), leading to the formation of a reactive species known as peroxymonocarbonate. This relationship not only underscores the connection between environmental changes and human physiology but also highlights an area of science that has historically received little attention: the biochemical implications of increased CO2 levels.
The Formation of Peroxymonocarbonate in Cells
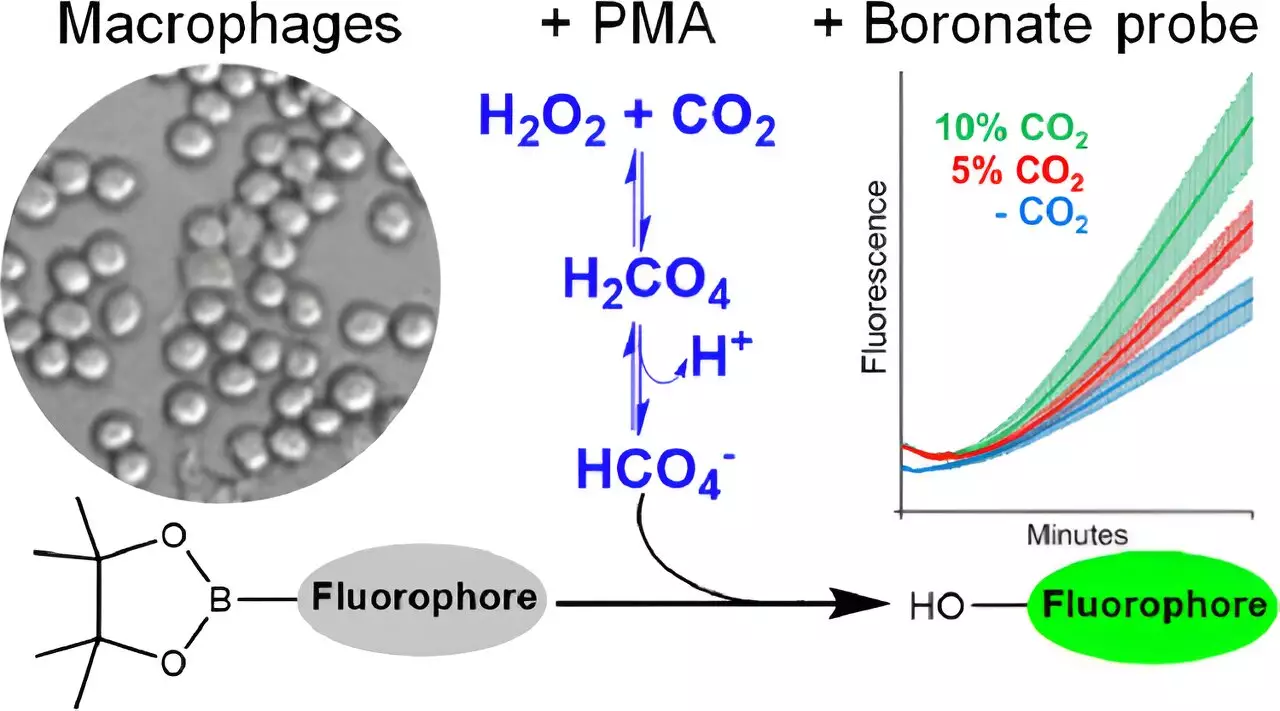
Peroxymonocarbonate may not be a household term, but its origins and consequences are pivotal in understanding oxidative stress and redox signaling in cells. Research conducted by a team at the University of São Paulo, led by Professor Ohara Augusto, revealed a novel technique for detecting this substance within cellular environments. Using innovative fluorescent molecular probes, the scientists successfully established proof of peroxymonocarbonate’s existence in living cells for the first time.
The study’s methodology involved a well-engineered enzyme reaction to generate physiological levels of hydrogen peroxide while simultaneously measuring the fluorescent reaction of boronate probes. These probes are crucial because they bind selectively with various reactive oxygen species, enabling researchers to distinguish the dynamics of oxidative molecules within cells. Notably, the experiment utilized activated macrophages—immune cells known for their robust role in generating oxidants—allowing for a clear demonstration that peroxymonocarbonate forms under specific conditions influenced by CO2 levels.
The implications of detecting peroxymonocarbonate extend far beyond academic curiosity. As a potent oxidant, peroxymonocarbonate plays a vital role in redox signaling, influencing how cells perceive and respond to oxidative stress. When cells encounter elevated levels of reactive species, including hydrogen peroxide, they initiate adaptive mechanisms to counteract potential damage. This includes the expression of genes that code for antioxidant enzymes, which help maintain the delicate balance within cellular environments.
However, an important aspect of this adaptation process is the rate at which oxidants act on proteins. Remarkably, peroxymonocarbonate oxidizes thiol proteins faster than hydrogen peroxide, suggesting that it could be a dominant player in mediating cellular responses to stress. This oversight may force scientists to reconsider existing theories about redox signaling pathways and emphasize the need for more extensive research on the biological roles of peroxymonocarbonate.
Understanding the Toxicity of Elevated CO2 Levels
The lack of attention to the physiological ramifications of rising CO2 levels is concerning, especially considering the epidemiological evidence linking urban CO2 concentrations to numerous health issues. The toxicity of CO2—often dismissed as a mere byproduct of respiration—deserves an in-depth exploration of how it alters biological functions.
Professor Augusto emphasizes that while CO2 is a natural component of our atmosphere and essential for biological processes, an excess can disturb various physiological pathways. Elevated CO2 not only modulates the reactivity of hydrogen peroxide and peroxynitrite but may also affect gene expression across various systems involved in inflammation and other cellular responses. This interrelationship forms a complex web of interactions that can lead to detrimental effects on human health, raising the urgency for a more nuanced understanding of how increased CO2 contributes to these pathways.
While the research spearheaded by Professor Augusto and his team has shed light on the existence of peroxymonocarbonate within cells, it also signals the beginning of a larger conversation. As peroxymonocarbonate was historically overlooked due to its elusive nature, its newly recognized biological significance positions it as a critical focus for future scientific inquiries. Understanding its formation and effects can provide insights into why certain cellular responses may occur and how they relate to environmental changes.
As climate change continues to pose challenges to global health, this line of inquiry will be essential in unraveling new mechanisms by which increasing atmospheric CO2 affects not only the environment but also human physiology. Given this interdisciplinary opportunity, there is a pressing need for collaboration among chemists, biologists, and environmental scientists to explore the multifaceted impact of CO2 and its downstream effects on cellular health. Understanding this dynamic may ultimately lead to more informed approaches to mitigating the consequences of climate change while safeguarding our biological systems.

Leave a Reply