Recent research spearheaded by Jason Hafner and his team at Rice University has unveiled new complexities surrounding the role of cholesterol within cell membranes. Published in the esteemed Journal of Physical Chemistry, this study is poised to enhance our understanding of how cholesterol not only contributes to the structural integrity of biomembranes, but also influences the functionality of the receptors embedded within these vital cellular components.
Cholesterol is a crucial lipid that plays a multifaceted role in maintaining the architecture of biological membranes. These membranes are intricate assemblies of proteins and lipids that regulate numerous cellular functions. By organizing membrane microdomains, cholesterol facilitates critical processes, including signal transduction and the localization of membrane proteins. The researchers emphasize that a deeper understanding of cholesterol’s interactions could elucidate the mechanisms behind various diseases, particularly those associated with cell membrane disarray, such as cancer and metabolic disorders.
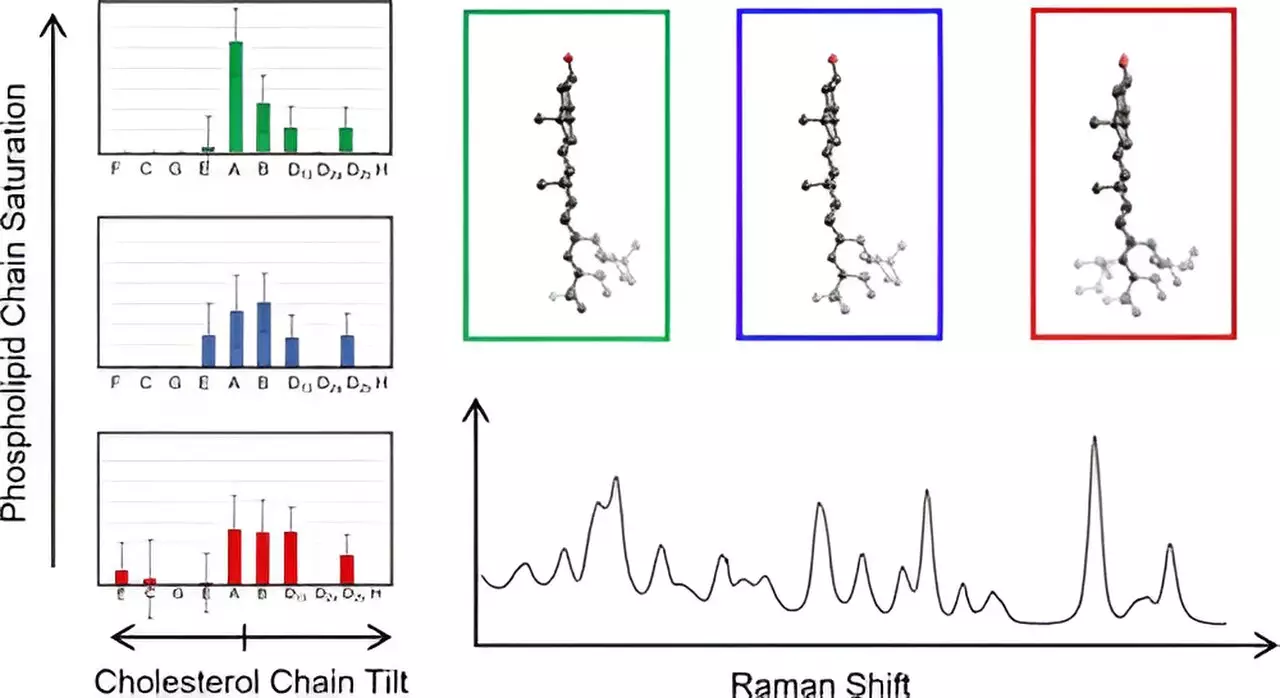
Despite its significance, elucidating the structural characteristics of cholesterol and its interactions within biomembranes has proven to be a formidable challenge for scientists. Traditional methods have struggled to yield clear insights regarding the molecular intricacies of cholesterol within these complex environments. This study by Hafner’s team represents a breakthrough, as it employs cutting-edge Raman spectroscopy to methodically explore the vibrational profiles of cholesterol molecules integrated within their natural membrane context.
Raman spectroscopy serves as a powerful analytical tool that leverages laser-induced light scattering to produce detailed vibrational spectra, revealing intricate information at the molecular level. This innovative approach allowed the research team to scrutinize cholesterol within biological membranes, aligning the gathered spectral data with predictive models derived from density functional theory—an advanced computational method prevalent in quantum mechanics. The synthesis of experimental readings and theoretical predictions showcased a remarkable correlation, deepening the understanding of cholesterol’s molecular behavior.
The research findings are groundbreaking, demonstrating for the first time how different cholesterol structures can be categorized based on the orientation of their carbon chains in relation to their fused ring structure. Hafner noted an unexpected consistency in the low-frequency spectra of all cholesterol molecules within similar groupings, suggesting a uniformity in certain structural features. This simplification not only enhances the analysis process but also provides a framework for future studies focused on membrane cholesterol dynamics, which are vital for understanding cellular functions and pathological states.
The study, which includes contributions from diverse researchers within the Rice University community, sets the stage for further explorations into the role of cholesterol in cell membranes. As more is understood about these molecular interactions, it may lead to discoveries that transform our approaches to tackling diseases that stem from membrane dysfunction. Understanding the subtleties of cholesterol’s role could open new avenues in the development of targeted therapies and innovative treatments, emphasizing the significance of this research within the broader field of biochemical sciences. The implications of these findings could indeed be far-reaching, influencing not only academic research but also clinical applications in disease prevention and management.

Leave a Reply