Recent scientific discoveries have shed light on the intricate world of single-celled organisms, particularly bacteria and archaea, revealing that they possess a variety of histones—proteins crucial for the structuring and organization of DNA. Historically thought to be present only in complex multicellular life forms, the identification of these proteins in simpler organisms marks a significant advancement in our understanding of cellular biology. A recent study led by Leiden Ph.D. candidate Samuel Schwab has unveiled a staggering 17 different groups of histones, each characterized by unique structural and functional properties, as published in the journal *Nature Communications*.
DNA is fundamentally a large molecular structure that poses challenges in spatial organization within the confines of cellular membranes. This is where histones come into play. By facilitating the compaction of DNA, histones form structures known as nucleosomes, around which DNA is meticulously wrapped. Their significance cannot be overstated; without these proteins, the functional organization of genetic material would be impractically chaotic. The identification of histones beyond more complex life has prompted researchers to rethink how the simplest life forms manage their genetic makeup.
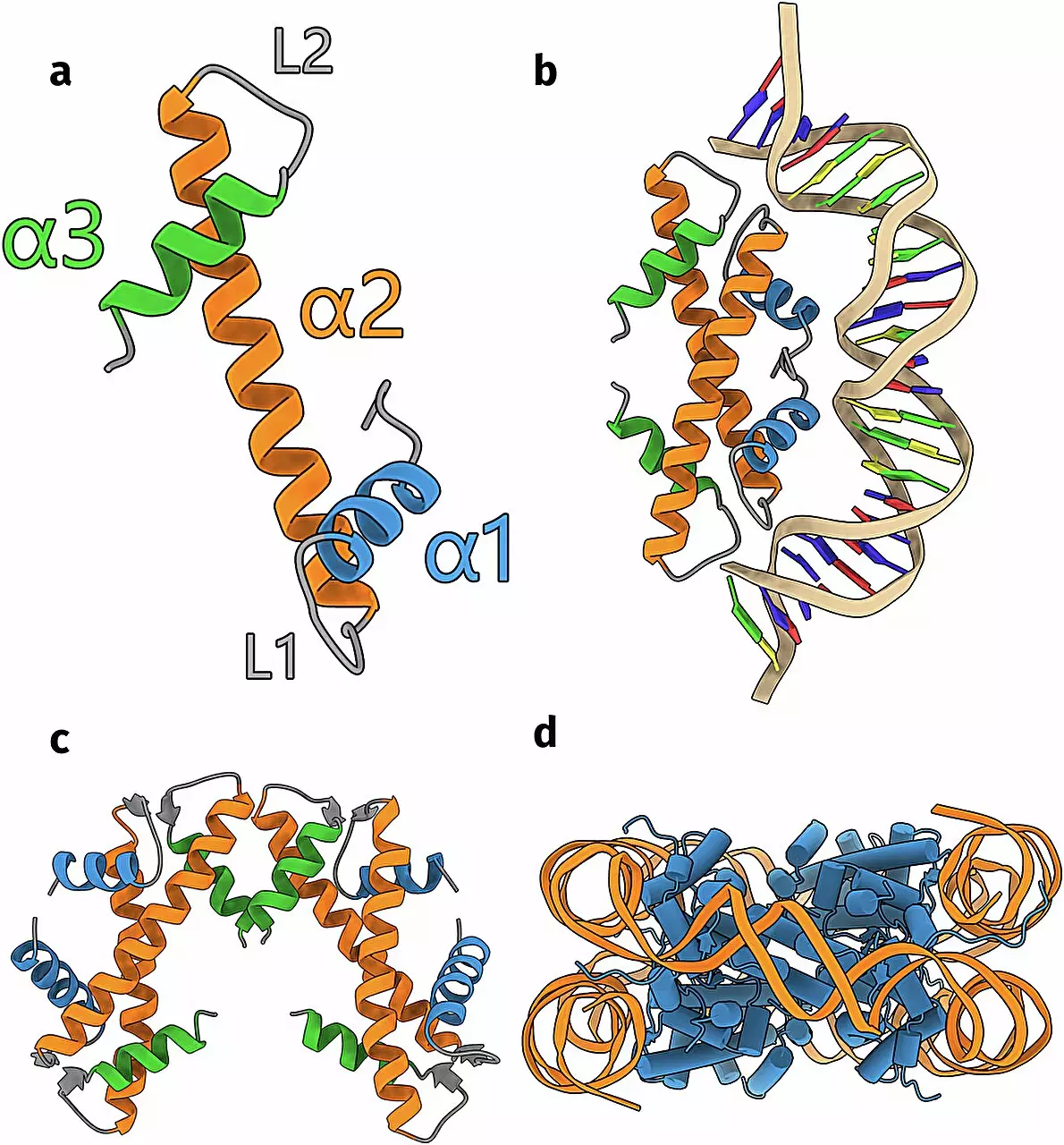
The pioneering work of Schwab and his colleagues utilized advanced computational tools, particularly the AI algorithm AlphaFold. This state-of-the-art program is capable of predicting protein structures from their corresponding DNA sequences. Schwab’s research began with an extensive database that included approximately 6,000 DNA sequences potentially coding for undiscovered histones in bacteria and archaea. Using the capabilities of AlphaFold, Schwab’s team successfully predicted the structures of these histones, unlocking insights into their diversity.
To affirm their computational predictions, Schwab’s team engaged in empirical testing, analyzing one of the novel histone structures in the laboratory. Remarkably, the laboratory findings corroborated the predictions, demonstrating the potential reliability of AI in biological research. This milestone not only highlights the accuracy of the predictions but also emphasizes the value of integrating technology with traditional laboratory techniques.
One of the most intriguing facets of Schwab’s findings is the variability in histone structures. The existence of 17 distinct groups not only suggests a rich evolutionary history but also raises questions about the functional dynamics these proteins hold. The structural diversity implies a range of interactions with DNA, including some histones that may orchestrate looping mechanisms or facilitate the folding of DNA strands. This complexity suggests that these proteins may be involved in processes beyond mere DNA packaging.
Moreover, Schwab’s research unveiled that some histone variants might bind to cellular membranes instead of DNA, indicating a potential functional role unrelated to DNA organization. This disappointing revelation signifies that histones may have additional roles in cellular processes that warrant further investigation.
The implications of this groundbreaking research are vast. Understanding the diverse roles of histones in single-celled organisms is pivotal for expanding our knowledge of genetic regulation across different life forms. Historical assumptions regarding the simplicity of single-celled organisms are being challenged, prompting a re-evaluation of our understanding of cellular biology. Moreover, the insights gained can significantly enhance our ability to interpret DNA-related data, leading to advancements in genetics, evolutionary biology, and possibly medical research.
Supervisor Remus Dame highlights the potential of this research to illuminate the complex mechanisms of DNA organization and enhance our comprehension of cellular function and evolution. Schwab echoes this sentiment, emphasizing that the journey into understanding histones is far from over, with numerous avenues still await exploration.
The discovery of diverse histone structures in single-celled organisms marks a paradigm shift in the study of molecular biology. Schwab’s research not only demonstrates the critical functions of histones in DNA organization but also opens up new avenues of inquiry into how even the simplest life forms manage complex biological processes. As researchers continue to investigate these proteins’ roles, we are reminded of the intricate complexities that underpin life at all levels, offering profound implications for science and our appreciation of biological diversity.

Leave a Reply