The concept of self-assembly in chemistry draws intriguing parallels to our everyday frustrations, such as attempting to piece together complex IKEA furniture. Instead of enduring hours of struggle, imagine if these pieces could autonomously organize themselves into a functional form. In the realm of biosystems, nature often achieves such assembly through the remarkable properties of molecular interactions. This fascinating aspect of supramolecular chemistry is crucial for understanding the formation of structures like proteins and viruses. This article delves into the discoveries made by researchers at Osaka University, unlocking new possibilities in material science.
Unraveling the Mechanisms of Self-Assembly
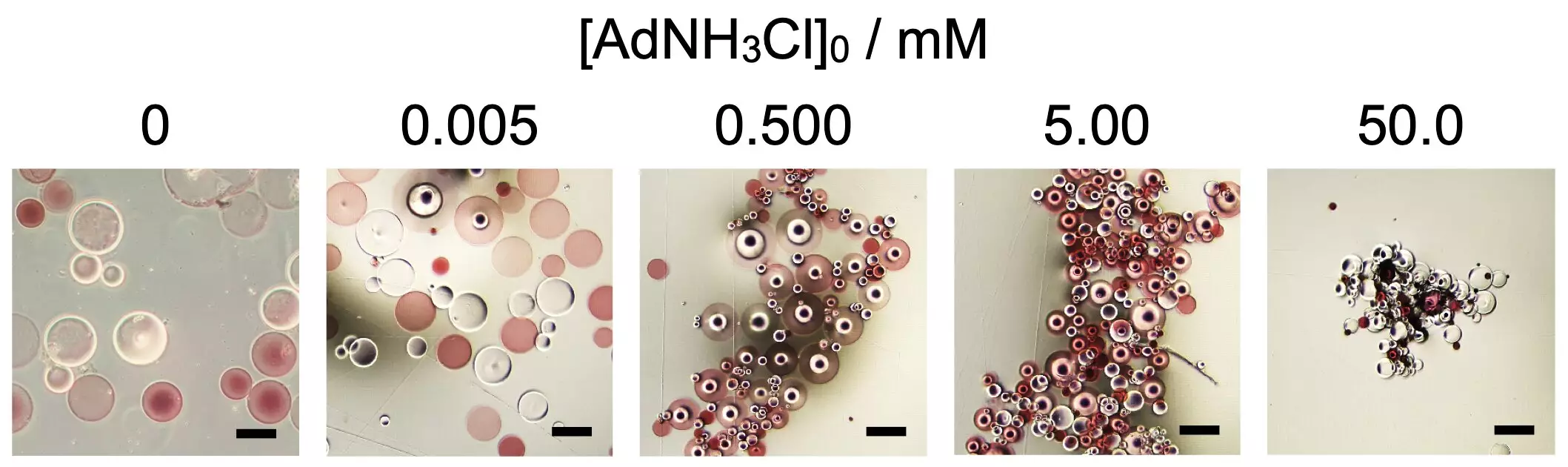
At the heart of this research lies poly(sodium acrylate), a super absorbent polymer that demonstrates remarkable self-assembly capabilities under certain conditions. The Osaka University team’s findings, recently published in *Scientific Reports*, reveal that the proper concentration of an additive known as 1-adamantanamine hydrochloride—crucially, the presence of a specific threshold—is mandatory for the successful assembly of spherical microparticles. The researchers borrowed principles from biological systems, illustrating that just as proteins derive their structure from sequences of amino acids, polymers can also be designed to interact and assemble in complex ways.
The study showcased intricate modifications involving functional groups like β-cyclodextrin and adamantane. Each of these unique chemical groups plays a pivotal role in dictating the nature of interactions among the polymer chains. This tuning potential could pave the way for the creation of advanced “smart materials” that can adapt their form and function based on environmental changes, such as temperature fluctuations or the introduction of new chemicals.
Applications and Future Implications
The implications of this research stretch far beyond the laboratory, offering revolutionary insights into materials science and biology. By manipulating polymer configurations, engineers and scientists can potentially devise new materials that change shape and function in response to external stimuli. This capability could serve as a foundation for responsive systems in robotics, biomedical devices, or even environmentally responsive construction materials.
Additionally, understanding these molecular assemblies may provide insights into the fundamental principles that govern biological forms and structures. Lead study author Akihito Hashidzume posits that “all living organisms are just collections of supramolecular polymers with sophisticated functions.” Such a perspective invites broader discussions surrounding evolutionary biology and the origins of life, one that examines how simple molecular interactions can give rise to complex biological systems.
Overall, the insights garnered from the self-assembly of spherical microparticles underscore the elegance of supramolecular chemistry. By comprehending how microscopic interactions dictate macroscopic structures, researchers are moving closer to uncovering the mysteries of both synthetic and biological materials. As we continue to explore the expansive possibilities within this field, the hope remains that innovations in smart materials and bioinspired designs will profoundly enhance various industries, merging the realms of chemistry and biology in unprecedented ways.

Leave a Reply