Hydrogen energy is gaining recognition as a pivotal player in the global quest for sustainable energy solutions. Its clean-burning nature emits only water when utilized, positioning it as an ideal alternative to fossil fuels. As nations grapple with the consequences of climate change and seek to reduce carbon footprints, hydrogen energy emerges not just as a viable option but as a vital component in achieving energy independence and environmental sustainability. However, unlocking hydrogen as an efficient energy source demands innovation, particularly in the production methods that harness hydrogen’s potential. Water splitting, an electrochemical process, offers one such pathway by converting water into hydrogen and oxygen.
Despite its promise, the water-splitting process is not without its challenges. The Oxygen Evolution Reaction (OER), which occurs at the anode during water splitting, is particularly cumbersome. The slow kinetics associated with OER impede the overall efficiency of hydrogen production, necessitating the development of advanced catalysts. These catalysts play a crucial role in enhancing the reaction rates, ensuring that water splitting can efficiently contribute to hydrogen generation. In this context, the exploration of novel catalyst materials is not only timely but essential for the maturation of hydrogen energy technologies.
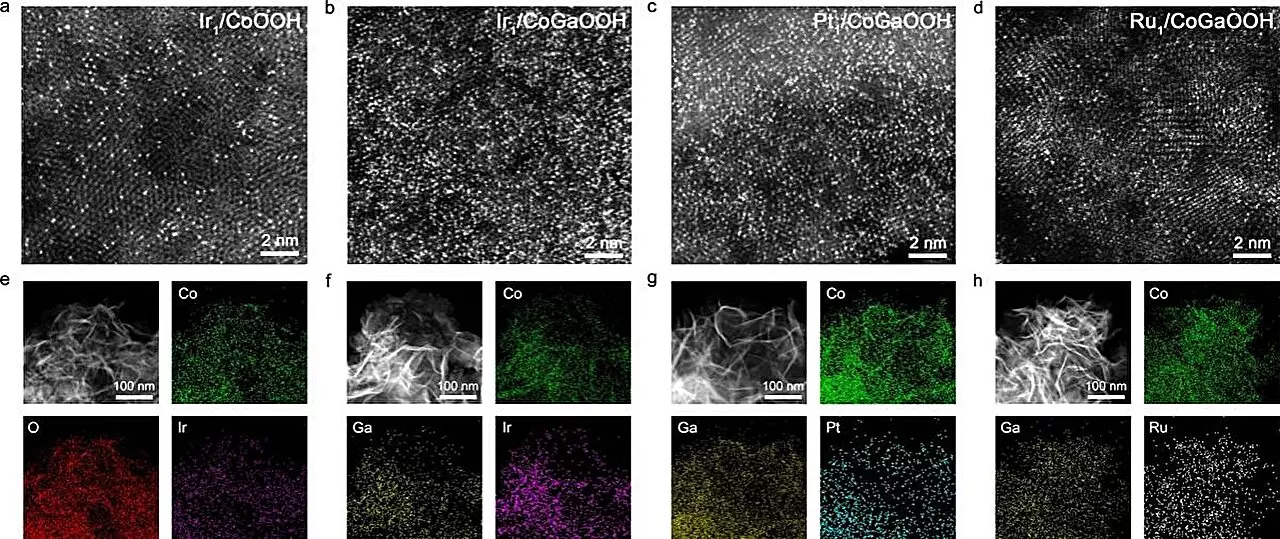
Recent advancements in catalyst technology have provided a glimmer of hope. A team led by Prof. Bao Jun at the University of Science and Technology of China (USTC) made significant strides in surmounting the challenges posed by OER. Their investigation into cobalt-based, oxide-supported single-atom catalysts (SACs) revealed remarkable performance characteristics attributable to their unique structural features. SACs have attracted considerable attention due to their ability to exhibit enhanced catalytic activity when engineered for optimal atomic density.
The study published in *Angewandte Chemie* delves deeply into the influence of atomic arrangement on catalytic performance. As the density of individual catalytic atoms on a surface increases, the interactions among these neighboring atoms—referred to as neighboring synergy—become crucial. By refining the distribution of single atoms, the research team was able to improve how reaction intermediates are adsorbed, ultimately amplifying the catalysis process.
To achieve high-density single atom formation, the researchers ingeniously incorporated gallium (Ga) atoms into the cobalt-based oxide lattice. This innovative synthesis method allowed for fine-tuning of the electronic architecture surrounding the iridium (Ir) single atoms, effectively enhancing bond strengths with oxygen defect sites. The results were compelling: the high-density Ir single-atom catalyst, designated as Nei-Ir1/CoGaOOH, demonstrated a remarkable overpotential of only 170 mV at a current density of 10 mA cm^-2, coupled with stable operations over extended durations—exceeding 2000 hours of testing.
Moreover, the catalyst’s robustness was further showcased by its ability to maintain functionality at a current density of 1 A cm^-2 for over 50 hours. The in situ Raman spectroscopic analyses provided corroborative evidence of the catalyst’s structural stability under operational conditions, reiterating its potential for practical applications.
Diving deeper into the mechanisms that underpin such advancements, the research team found that performance improvements stem not solely from modifications to electronic structure, but rather from the synergistic interactions between high-density Ir atoms. These interactions notably stabilize reaction intermediates like *OOH* through additional hydrogen bonding. This stabilization effect is pivotal; it lowers the energy barriers associated with the OER, thereby facilitating a more efficient reaction process.
The findings from Prof. Bao Jun and his team’s research mark a significant milestone in the development of electrochemical catalysts for hydrogen production. By elucidating the underlying mechanisms that enhance catalyst performance, this work paves the way for further innovations in the field. As hydrogen energy continues to evolve, studies that focus on atomic-level interactions will be instrumental in realizing its full potential. The implications extend beyond mere academic interest—they represent concrete steps toward a cleaner, more sustainable energy future. This research serves as a beacon, signaling that the era of hydrogen energy is on the horizon, propelled by cutting-edge scientific inquiry and ingenuity.

Leave a Reply