The oxygen evolution reaction (OER) is a critical process in the quest for sustainable energy solutions, particularly in water splitting and metal-air batteries. These technologies are essential for harnessing renewable energy resources, such as solar and wind, which can be intermittent. However, the efficiency of OER has long been impeded by slow reaction kinetics that demand the presence of high-performance, stable catalysts. Recent research has made promising strides in addressing this challenge, suggesting a bright future for clean energy applications.
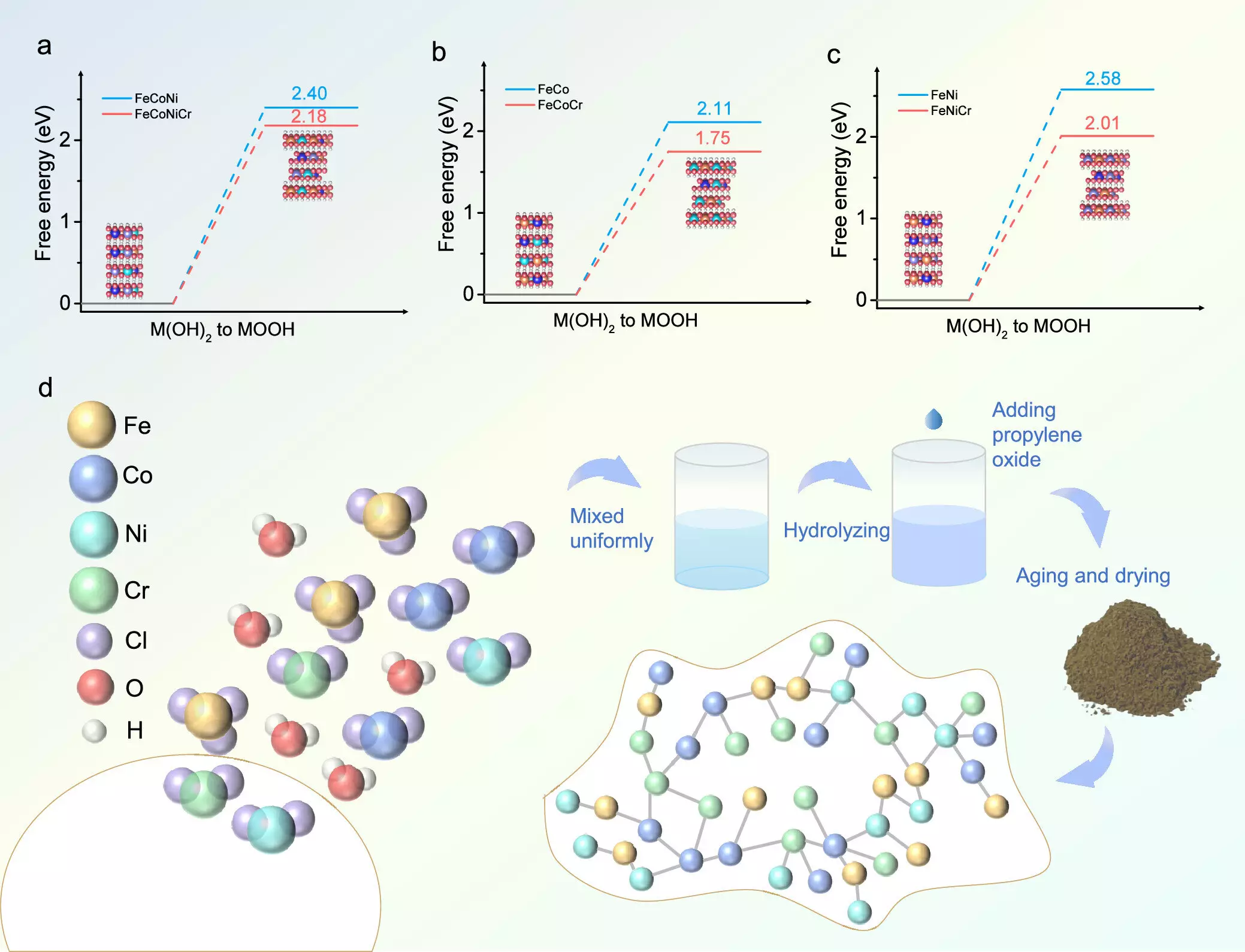
A team of researchers recently published groundbreaking findings in the journal ACS Catalysis, shedding light on a novel approach to catalyst design. By integrating chromium (Cr) into transition metal hydroxides, the group successfully enhanced the catalytic activity necessary for OER. Utilizing a combination of density functional theory (DFT) calculations and experimental methodologies, the researchers synthesized a multi-functional catalyst known as FeCoNiCr hydroxide. This catalyst was produced through an aqueous sol-gel method that guarantees a homogenous distribution of its constituent elements, which is vital for consistent performance.
Performance Metrics and Experimental Outcomes
The operational efficacy of the newly developed FeCoNiCr catalyst was remarkable. In alkaline environments, it displayed an impressively low overpotential of 224 mV, outperforming analogous catalysts by a substantial margin of 52 mV. Furthermore, durability tests revealed that the catalyst could sustain its performance for more than 150 hours of continuous use. Another notable application of this catalyst was in a zinc-air battery, which operated smoothly for 160 hours with minimal voltage discrepancies during discharge and charge cycles.
The researchers have delved deeply into the underlying mechanisms that contribute to the enhanced catalytic performance of Cr-doped metal hydroxides. According to lead researcher Hao Li from Tohoku University’s Advanced Institute for Materials Research, chromium significantly accelerates the phase transition of metal hydroxides to an active oxyhydroxide phase, thereby improving OER kinetics. DFT calculations elucidated that the introduction of Cr fine-tunes the electronic states of the catalyst’s active sites, optimizing the adsorption energies of reaction intermediates, and thus enhancing overall reaction efficiency.
Future Directions and Implications
Looking ahead, the research team intends to broaden the scope of their study by investigating the effects of additional elements on the catalytic properties. Di Zhang, an assistant professor at WPI-AIMR, emphasized the importance of this research for future advancements in clean energy technologies. The methodologies established in this study serve as a foundation for quickly screening various materials, paving the way for the design of even more efficient and resilient catalysts.
As global energy demands shift toward sustainability, the discovery and refinement of effective catalysts are essential. The innovations presented in this research not only enhance the efficiency of hydrogen production but also contribute significantly to the broader landscape of renewable energy technologies. The pursuit of cost-effective catalytic solutions could therefore be instrumental in accelerating the transition to cleaner energy systems worldwide.

Leave a Reply