Hydrogen has long been considered a promising fuel for reducing greenhouse gases, particularly when produced through the splitting of water molecules using renewable energy sources. However, this process is far from simple due to the complex chemistry involved. The key to efficiently breaking water into hydrogen and oxygen lies in the use of catalysts, which act as chemical “deal makers” to facilitate the necessary reactions. The U.S. Department of Energy’s Brookhaven National Laboratory and Columbia University have recently made significant strides in this field by developing a highly efficient catalyst for the oxygen evolution reaction.
Designing a New Catalyst
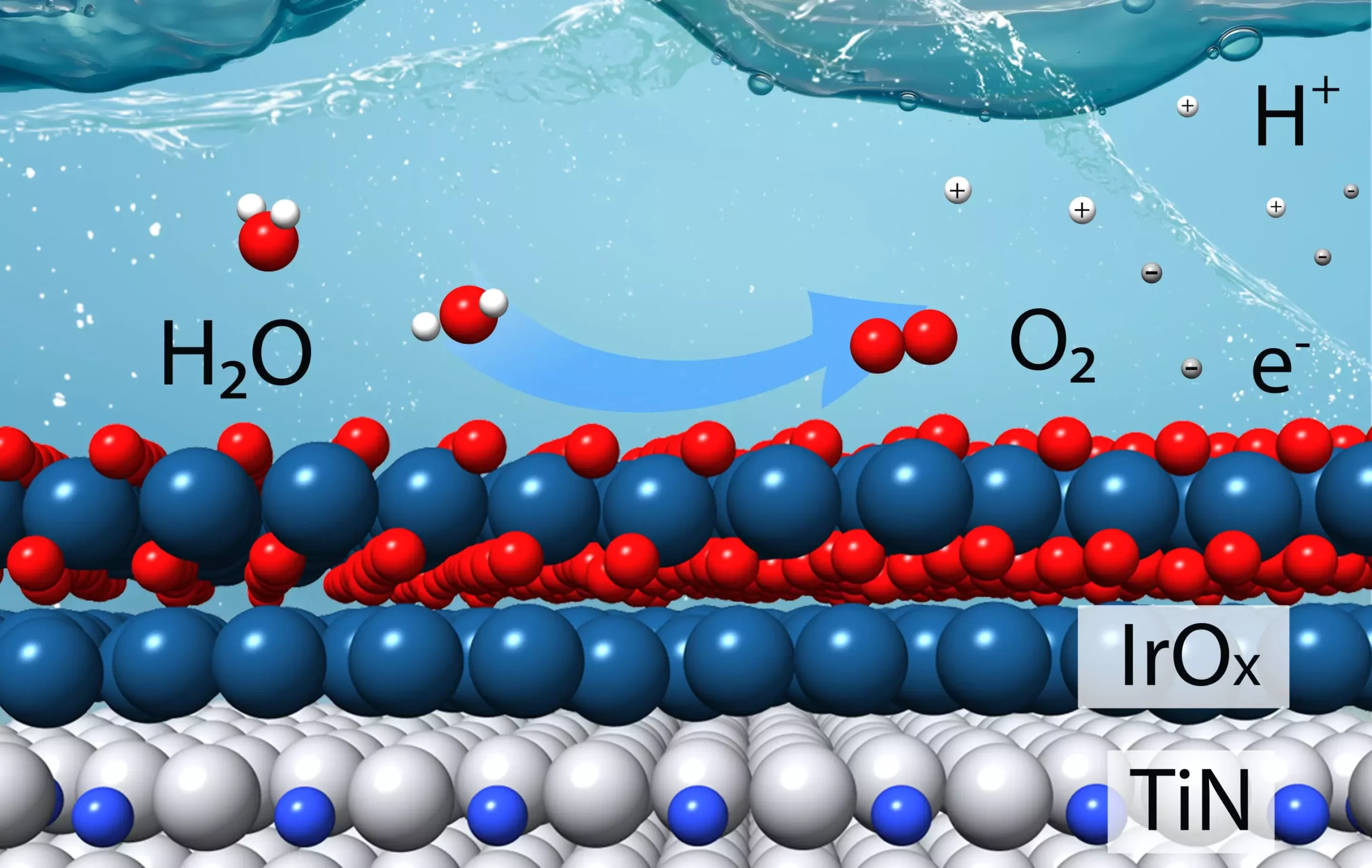
The catalyst, which was designed based on theoretical calculations, aims to minimize the use of iridium, an expensive metal commonly used in catalytic materials, while maximizing stability in acidic conditions. The research team’s innovative approach involved creating models of the catalyst and conducting lab tests to validate their predictions. The results of these experiments exceeded expectations, demonstrating that the new catalyst is four times more effective than the state-of-the-art iridium catalyst currently available commercially.
Iridium plays a crucial role in the oxygen evolution reaction, providing the necessary active sites for separating hydrogen ions and oxygen. However, the scarcity and high cost of iridium make it an impractical choice for large-scale applications. By reducing the amount of iridium required in the catalyst, researchers hope to overcome this limitation and pave the way for widespread adoption of hydrogen production technologies.
The process of designing the new catalyst involved intricate theoretical calculations to determine the optimal configuration of iridium on the catalyst’s surface. By leveraging density functional theory calculations and advanced computing resources, the researchers were able to model the behavior of different iridium layers on a titanium nitride core. This information guided the experimental team in creating catalyst samples for evaluation, ultimately leading to the successful development of a highly effective catalyst.
The groundbreaking research on hydrogen production catalysts opens up new possibilities for scaling up green hydrogen production. While challenges remain in terms of production scalability and powder consistency, the guidelines provided by this study offer valuable insights for industrial chemists. By developing core-shell structures with a uniform layer of iridium, scientists can work towards lowering the cost of water splitting and achieving large-scale production of green hydrogen in the future.

Leave a Reply