Basic oxide catalysts play a vital role in chemical synthesis for the production of chemicals, pharmaceuticals, and petrochemicals. To enhance the catalytic power of these catalysts, researchers have focused on improving their basicity. By improving the ability to donate electrons or accept hydrogen ions, these catalysts can become more efficient and effective in various chemical processes.
Different strategies have been deployed to improve the basicity of basic oxide catalysts. These strategies include:
– Doping the catalyst with highly electronegative cations like alkali metals.
– Substituting oxide ions with anions of different valences such as hydride (H-) or nitride (N3-) ions.
– Increasing the electron density in the catalyst by introducing oxygen vacancies next to oxide anions.
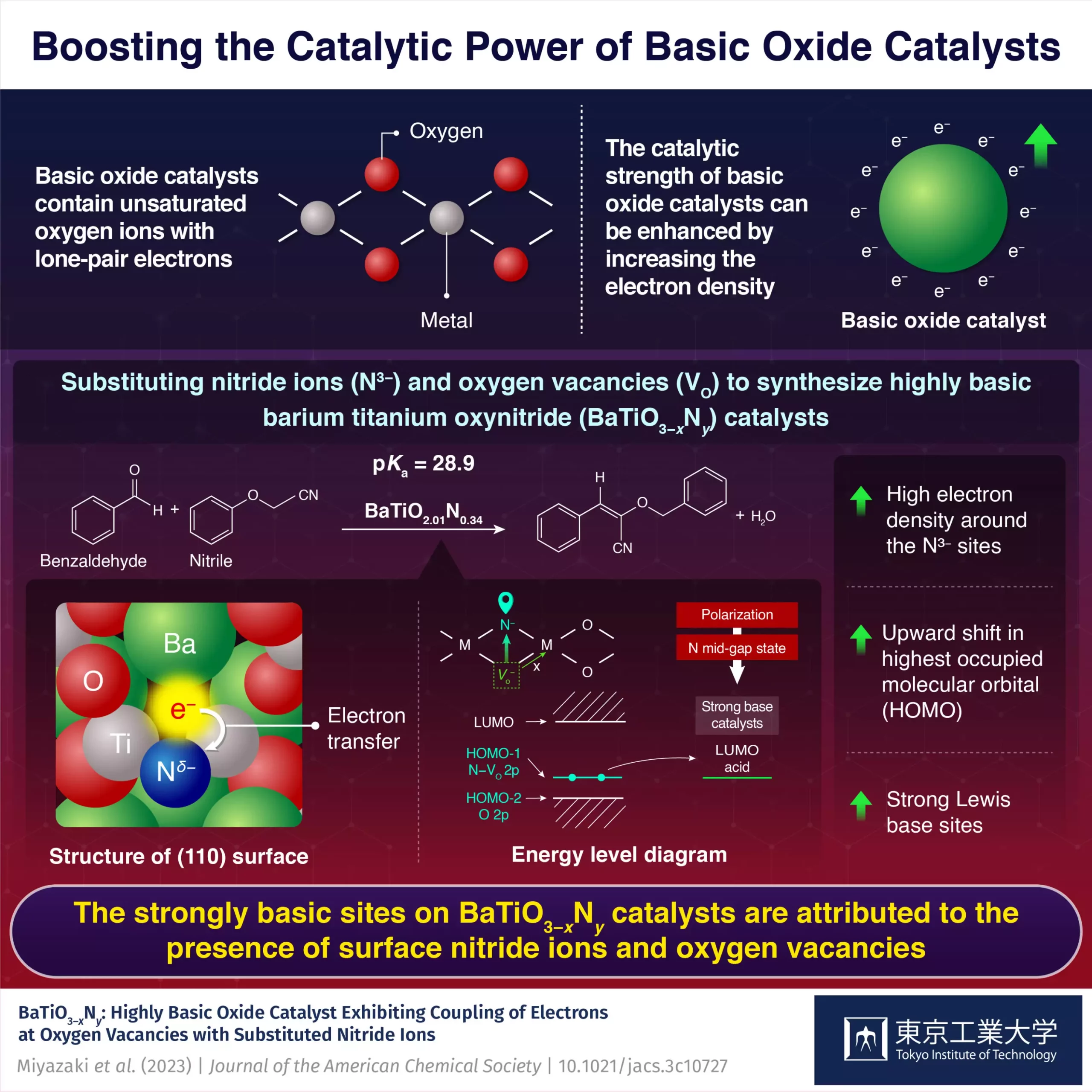
A recent study conducted by a team of researchers from the Tokyo Institute of Technology led by Assistant Professor Masayoshi Miyazaki has made significant progress in developing highly basic catalysts. The team developed a hexagonal BaTiO3-xNy oxynitride catalyst with basicity comparable to that of superbases.
The researchers achieved enhanced basicity by substituting nitride ions and introducing oxygen vacancies into face-sharing Ti2O9 dimer sites in BaTiO3-x. This substitution and introduction of vacancies changed the electronic structure of the catalyst, shifting the energy level of the highest occupied molecular orbitals (HOMO) upwards. The upward shift made it more favorable for electrons to be donated to a reactant’s lowest unoccupied molecular orbital (LUMO).
The introduction of oxygen vacancies adjacent to the doped nitride ions further increased the electron density, raising the HOMO energy level. As a result, the oxynitride catalyst became highly basic with a high tendency to donate electrons.
The coupling of substituted nitride ions to electrons at oxygen vacancies played a crucial role in improving the basicity of the oxynitride catalyst. This synergetic effect made the oxynitride catalyst more basic compared to other materials like BaTaO2N and LaTiO2N that do not contain oxygen vacancies.
The highly basic nature of the oxynitride catalyst facilitated Knoevenagel condensation reactions. This reaction involves the formation of a C-C bond between the carbonyl and methylene groups, with a basic catalyst accepting a proton from the methylene group. The researchers observed that the oxynitride catalyst BaTiO2.01N0.34 could accept protons from highly basic nitrile reactants with a pKa value as high as 23.8 and 28.9.
The ability of the catalyst to accept hydrogen ions from highly basic nitrile reactants demonstrates its basic strength, which is comparable to that of superbases. Superbases typically have pKa values around 26.
Furthermore, the oxynitride catalyst demonstrated excellent stability and maintained its catalytic activity even after repeated use. This stability makes it suitable for practical applications in various chemical processes.
The development of highly basic catalysts is crucial for improving the efficiency and effectiveness of chemical synthesis. This study by the researchers from the Tokyo Institute of Technology has laid the foundation for the advancement of basic oxide catalysts.
By substituting nitride ions and introducing oxygen vacancies, the researchers successfully enhanced the basicity of a hexagonal BaTiO3-xNy oxynitride catalyst. The synergetic effect of these modifications resulted in a highly basic catalyst with improved electron-donating abilities.
The oxynitride catalyst exhibited exceptional catalytic activity, specifically in Knoevenagel condensation reactions. Its stability and maintained catalytic performance after repeated use further demonstrate its potential for practical applications.
Moving forward, the combination of surface anion species and vacancies will be crucial in the synthesis of even more highly basic catalysts. This research opens up new possibilities for developing advanced catalysts for a wide range of chemical processes.

Leave a Reply