The use of lithium metal as an anode in batteries offers a promising solution for enhancing energy density compared to other materials. However, several challenges exist at the interface between the electrode and electrolyte, hindering the development of a safer and more functional battery system. Researchers have recognized the need to replace the graphite anode with a lithium metal anode to achieve higher energy density. Unfortunately, the natural solid-electrolyte interphase (SEI) formed between lithium metal and the electrolyte is brittle and fragile, resulting in poor performance and limited lifespan. Nevertheless, the advent of artificial solid electrolyte interphase (ASEI) brings hope for overcoming these issues and creating a more reliable and powerful source of energy for electric vehicles and other applications.
The current state of lithium metal batteries (LMBs) faces two main challenges: the formation of a solid-electrolyte interphase and dendrite growth during charging. The reactive nature of lithium metal leads to the formation of a passivation layer, the SEI, which affects battery performance. Moreover, dendrites, branch-like structures, can cause internal damage, leading to short-circuits and safety hazards. These weaknesses restrict the practicality of LMBs and necessitate the development of effective solutions.
To improve the lithium metal anode, researchers propose several strategies that can mitigate its limitations. Homogenizing the distribution of lithium ions can minimize deposits on negatively charged areas, reducing dendrite formation and preventing premature decay and short-circuits. Additionally, facilitating the diffusion of lithium ions while ensuring electrical insulation between layers maintains the structural and chemical integrity during battery cycling. It is crucial to reduce the strain at the electrode-electrolyte interface to ensure proper connectivity.
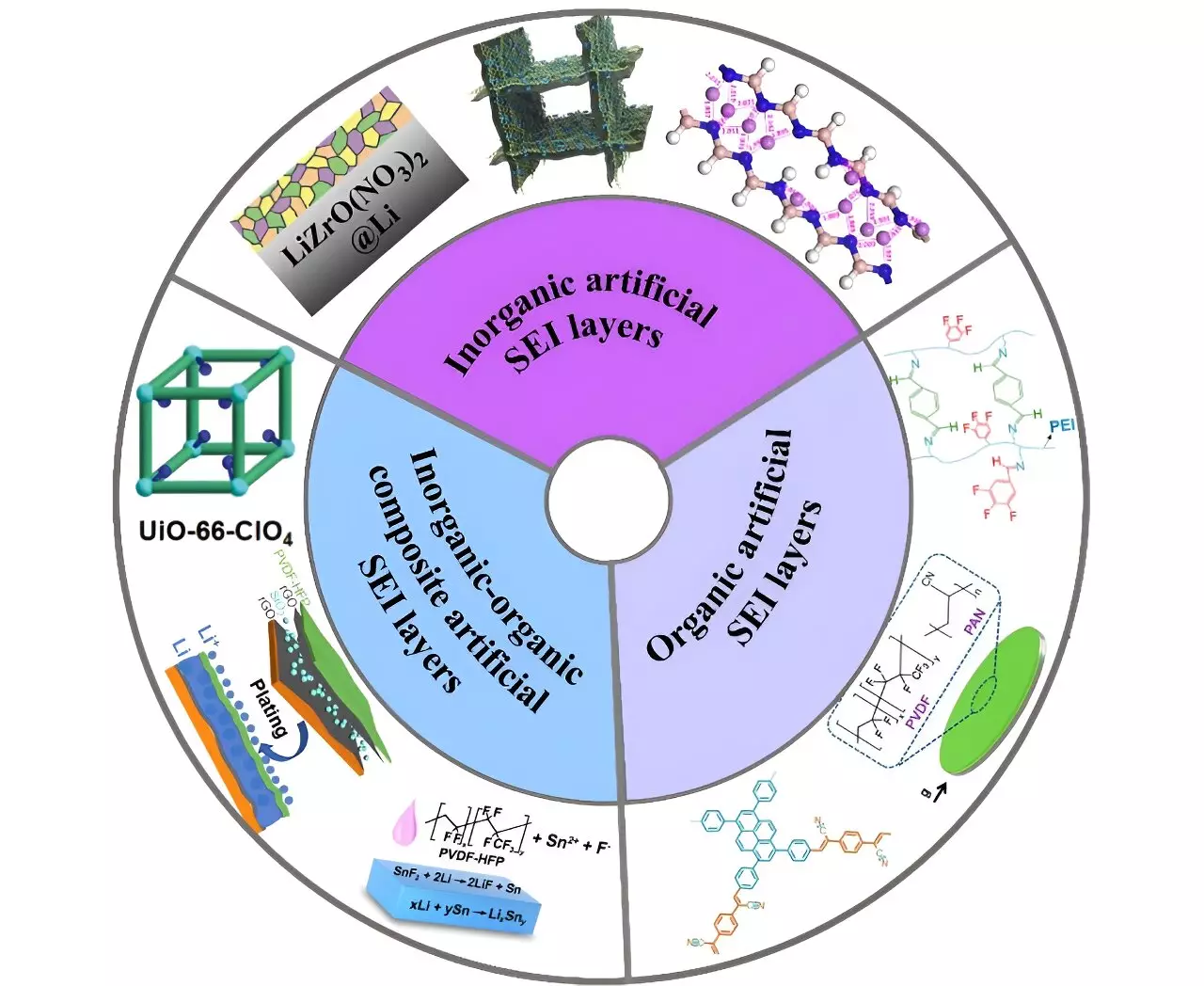
Among the strategies explored, polymeric ASEI layers and inorganic-organic hybrid ASEI layers show significant potential. Polymeric layers offer adjustable strength and elasticity, making them highly adaptable. Moreover, they possess functional groups similar to electrolytes, ensuring compatibility and enhancing overall performance. Inorganic-organic hybrid layers excel in reducing thickness and improving component distribution, ultimately enhancing battery performance.
While the future of ASEI layers appears promising, it is crucial to address a few key areas for further improvement. Enhancing the adhesion of ASEI layers to the metal surface is essential to optimize battery function and longevity. Stability in the structure and chemistry of the layers needs attention, as well as minimizing layer thickness to improve energy density. Overcoming these challenges is pivotal for the advancement of lithium metal batteries, paving the way for safer and more efficient energy storage.
The use of lithium metal as an anode for batteries offers the potential for higher energy density. However, the current limitations posed by the natural solid-electrolyte interphase and dendrite growth restrict the practicality of lithium metal batteries. The development of artificial solid electrolyte interphase (ASEI) layers provides a promising solution to mitigate these challenges. Strategies such as homogenizing lithium ion distribution and reducing strain at the electrode-electrolyte interface offer improvements in battery performance. Polymeric and inorganic-organic hybrid ASEI layers show great potential in enhancing the stability and functionality of lithium metal batteries. Further research is necessary to improve adhesion, stability, and thickness reduction in ASEI layers, ensuring the road ahead for improved lithium metal batteries is well-paved.

Leave a Reply