As the world continues its transition from conventional energy sources to greener alternatives, the focus on electricity as a power source expands beyond the realm of transportation. To truly create a sustainable future, the global manufacturing network must also adapt. In a recent study conducted by chemists at UChicago, a breakthrough in electrochemistry offers a way to use electricity to enhance chemical reactions, particularly in the synthesis of pharmaceutical drugs. This research, published in Nature Catalysis, not only contributes to our understanding of electrochemistry but also paves the way for designing and controlling reactions more efficiently, ultimately making them more sustainable.
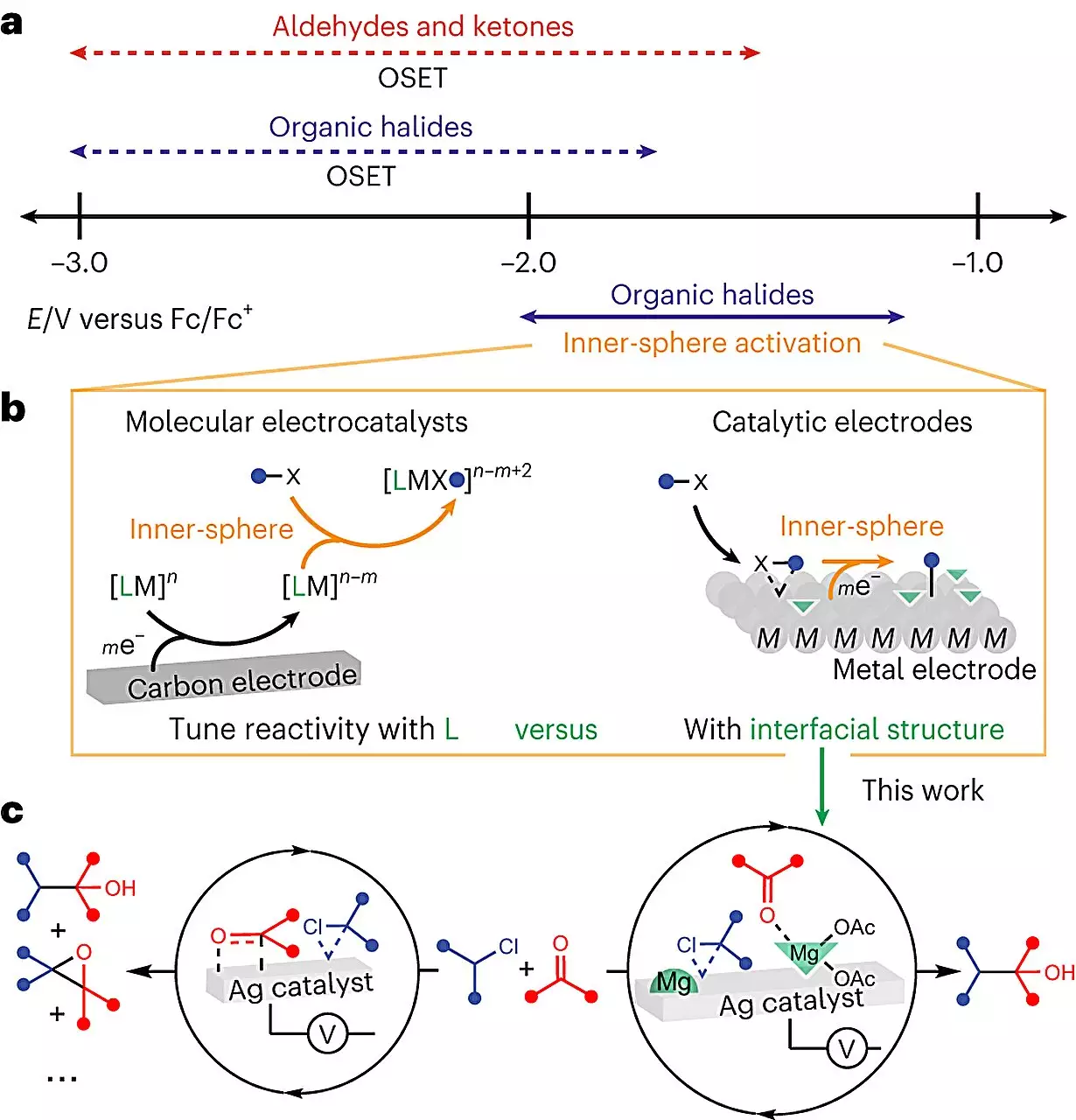
While electricity has the potential to increase the output of certain chemical reactions, the field of electrochemistry is far from straightforward. Molecular interactions during these reactions remain largely unknown, exacerbated by the introduction of a conductive solid, or electrode, to provide the necessary electricity. This complicates the process, making it challenging for scientists to decipher the individual roles of molecules and their specific order of interactions. However, instead of viewing this complexity as a hindrance, Anna Wuttig, the senior author of the paper and UChicago Neubauer Family Assistant Professor, sees it as an opportunity for unique design possibilities that are exclusive to electrochemistry.
Wuttig and her team focused their efforts on studying the surface of the electrode that facilitates the electrochemical reaction. Previous indications suggested that the surface itself serves as a catalyst, playing a significant role in the reaction. However, the challenge lay in understanding and controlling these molecular interactions at a more detailed level. The researchers decided to experiment with a commonly used reaction in the pharmaceutical industry, involving the formation of a bond between two carbon atoms. Theoretically, when electricity is employed in this reaction, the yield should be 100%, meaning all the initial molecules should transform into a single substance. However, actual laboratory experiments revealed a lower yield, indicating that the presence of the electrode was diverting some of the molecules away from their intended path during the reaction.
Optimizing Reaction Conditions
To overcome this obstacle, the team introduced a Lewis acid, a specific chemical compound, into the liquid solution. Remarkably, the Lewis acid helped redirect the molecules to the desired location, resulting in a near-complete conversion during the reaction. The researchers also utilized advanced imaging techniques to observe the reactions at the molecular level, gaining valuable insights into the interfacial structure. By visualizing and comprehending these intricate processes, they were no longer working with a black box, but rather grasping the inner workings of the reactions.
This progress marks a crucial step towards not only utilizing electrodes in chemistry but also predicting and controlling their effects. Moreover, the ability to reuse the electrode for multiple reactions contributes to the overall sustainability of the synthesis process. In most reactions, the catalyst dissolves in the liquid and is drained away during purification, resulting in a loss of resources. However, by implementing electrochemistry techniques, the team at UChicago envisions a future of sustainable synthesis, minimizing waste and optimizing efficiency.
As the world shifts towards a greener and more sustainable future, advancements in electrochemistry present unique opportunities for enhancing chemical reactions. The study conducted by UChicago chemists showcases the potential of electricity in boosting reaction yields, particularly in the synthesis of pharmaceutical drugs. Understanding and controlling molecular interactions at the electrode interface contributes to the wider goal of designing more sustainable chemical processes. By visualizing and comprehending these intricate processes, scientists move closer towards predictability. This research paves the way for a more efficient and environmentally friendly chemical industry, driving us towards a cleaner and brighter future.

Leave a Reply