Climate change and the rise of carbon dioxide emissions have pushed scientists to explore various methods for mitigating the effects of greenhouse gases. Direct air capture (DAC) is one such process aimed at achieving negative emissions by removing more carbon dioxide from the atmosphere than what is emitted. Scientists at the Department of Energy’s Oak Ridge National Laboratory have made significant progress in understanding a viable process for DAC using aqueous glycine, an amino acid known for its absorbent qualities.
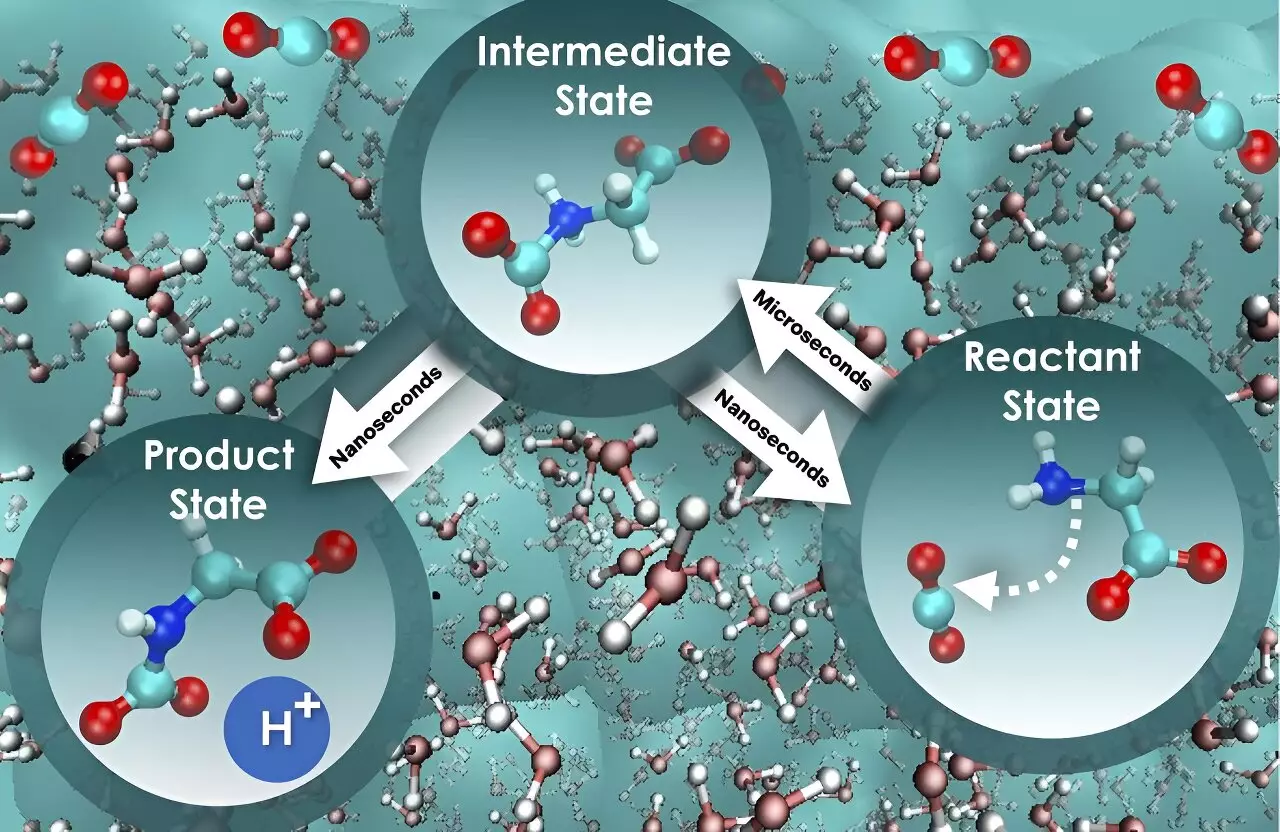
To fully comprehend the process of carbon dioxide sequestration, the researchers utilized advanced computational methods to investigate the dynamic interactions occurring in liquid solutions. They recognized the intricate dance between water molecules and chemicals, which can either marginally or significantly slow down a reaction. This fundamental understanding of nonequilibrium solvent effects proves essential in comprehending reaction kinetics. However, the researchers found that focusing solely on the free energy barrier, or the energy threshold necessary for a system to transition from one state to another, is an oversimplification that fails to provide the complete picture.
The study brought to light a more comprehensive approach that considers the influence of water on the motion along the reaction path. The researchers discovered that the initial step, where glycine interacts with carbon dioxide, is approximately 800 times slower in comparison to the subsequent step, where a proton is released to form a product state for holding the absorbed carbon dioxide. Surprisingly, the free energy barrier remains constant for both steps, highlighting the distinct speed of the critical stages and presenting new opportunities for boosting the efficiency of carbon dioxide absorption and separation.
While the study’s extensive ab initio molecular dynamics simulations provided valuable insights, they were limited by their short time and length scales and high computational costs. Recognizing these constraints, the researchers aim to combine machine learning approaches with highly accurate simulations to develop interatomic interaction potentials based on deep neural networks. This integration would enable molecular simulations at larger scales and significantly reduce computational costs, ultimately allowing for a more thorough understanding of DAC and its relationship with macroscopic factors such as temperature, pressure, and viscosity.
The findings of this research shed light on the intricate workings of DAC and underscore the crucial role of kinetics, thermodynamics, and molecular interactions in carbon dioxide sequestration using aqueous amino acids. As our understanding of these mechanisms deepens, the prospects of deploying a large-scale DAC technology become increasingly viable. Worldwide, several DAC projects are underway at different stages of research, testing, and development. With continued scientific advancements and exploration of innovative approaches, the goal of achieving negative emissions and mitigating the effects of climate change moves closer within reach.

Leave a Reply