Water is a fundamental molecule that plays a crucial role in numerous scientific fields, including astrochemistry and corrosion of metals. Understanding the ionization of water is of utmost importance for physical chemists, as it not only influences biological processes and radiation chemistry but also promotes corrosion at the interface between water and metals. In a groundbreaking study, RIKEN chemists have successfully isolated an elusive structure involving two water molecules, shedding light on the intricate process of water ionization. This discovery has the potential to revolutionize our understanding of fundamental chemical processes and pave the way for further research in the field.
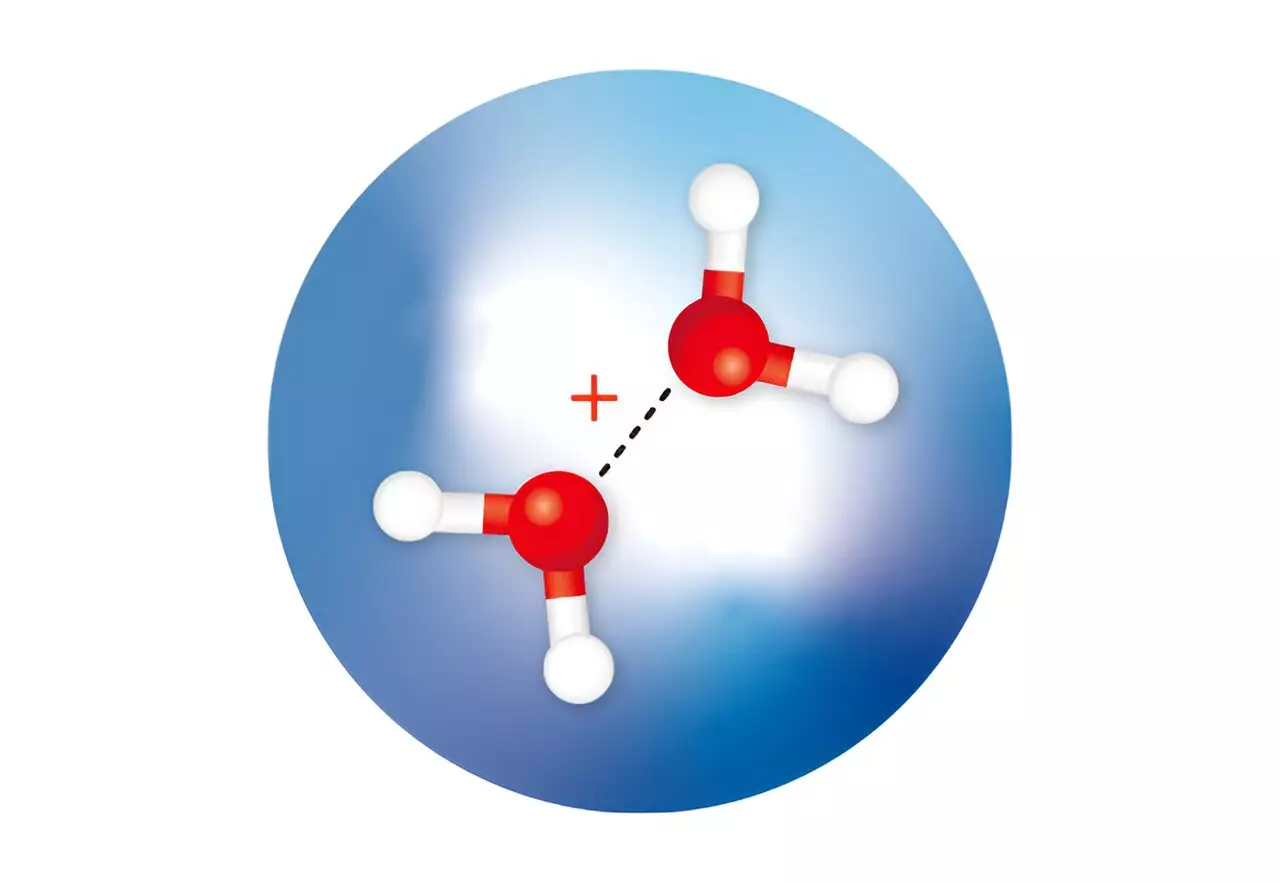
Previous calculations have predicted that following the ionization of a water molecule, two isomers of a positively charged ion of a water dimer would rapidly form. One isomer, known as H3O+·OH, has been observed and confirmed through experiments. It forms when a proton is transferred from one water molecule to another. However, the second isomer, with a half-bonded (or hemibonded) structure known as H2O·OH2+, has remained elusive, never being isolated or confirmed by spectroscopic measurements. This higher-energy isomer has been a lingering mystery in the field of physical chemistry.
Susumu Kuma and his team from the RIKEN Atomic, Molecular and Optical Physics Laboratory achieved a major breakthrough by successfully isolating both water dimer ions using an innovative approach. Utilizing tiny droplets of cold helium, the researchers trapped the elusive water dimer ions and analyzed their structures using infrared spectroscopy. The ultracold environment allowed for the rapid cooling of water molecules within the helium droplets, enabling the stabilization of the hemibonded isomer.
To confirm the presence and structure of the water dimer ions, Kuma and his team employed computational and spectroscopic methods. The spectroscopic signatures of the molecular ions were nearly identical to those of bare ions, indicating that they could directly compare the measurements on the bare ions with the results from quantum-chemical calculations. This breakthrough finding not only provides critical insights into the structural analysis of the dimers but also opens up new avenues for further studies in the field.
The discovery of the hemibonded water cations by RIKEN chemists is a significant milestone in the study of water ionization. It not only addresses the longstanding question about the existence of the elusive isomer but also paves the way for future investigations into primary events crucial for understanding the radiation chemistry of water. Susumu Kuma envisions that this breakthrough will fuel further studies and exploration of other yet-to-be-observed structures. His team plans to extend their research to examine larger water complex cations within helium droplets, offering exciting prospects for uncovering new and unexplored realms in this field of research.
RIKEN chemists’ successful isolation and structural analysis of the hemibonded water cations marks a remarkable achievement in the study of water ionization. This breakthrough not only provides valuable insights into the fundamental process of ionization but also offers a platform for future research and exploration. The implications of this discovery extend beyond the field of physical chemistry, with potential applications in astrochemistry, corrosion of metals, and radiation chemistry. As scientists delve deeper into the mysteries of water, they may uncover even more elusive structures and mechanisms that shape our understanding of the natural world.

Leave a Reply