Non-reciprocal interactions are a fascinating aspect of nature that defy the conventional laws of reciprocal forces such as gravity and electromagnetism. These interactions play a crucial role in the complex behavior exhibited by living organisms. While the mechanisms behind non-reciprocal interactions in microscopic systems like bacteria have been previously explained by external forces, a recent study conducted by researchers from the University of Maine and Penn State has shed new light on the subject.
The research published in Chem presents a groundbreaking finding on how single molecules can interact non-reciprocally without the need for hydrodynamic effects. The study, led by theoretical physicist R. Dean Astumian from UMaine, in collaboration with Ayusman Sen and Niladri Sekhar Mandal from Penn State, introduces a different mechanism by which non-reciprocal interactions can occur.
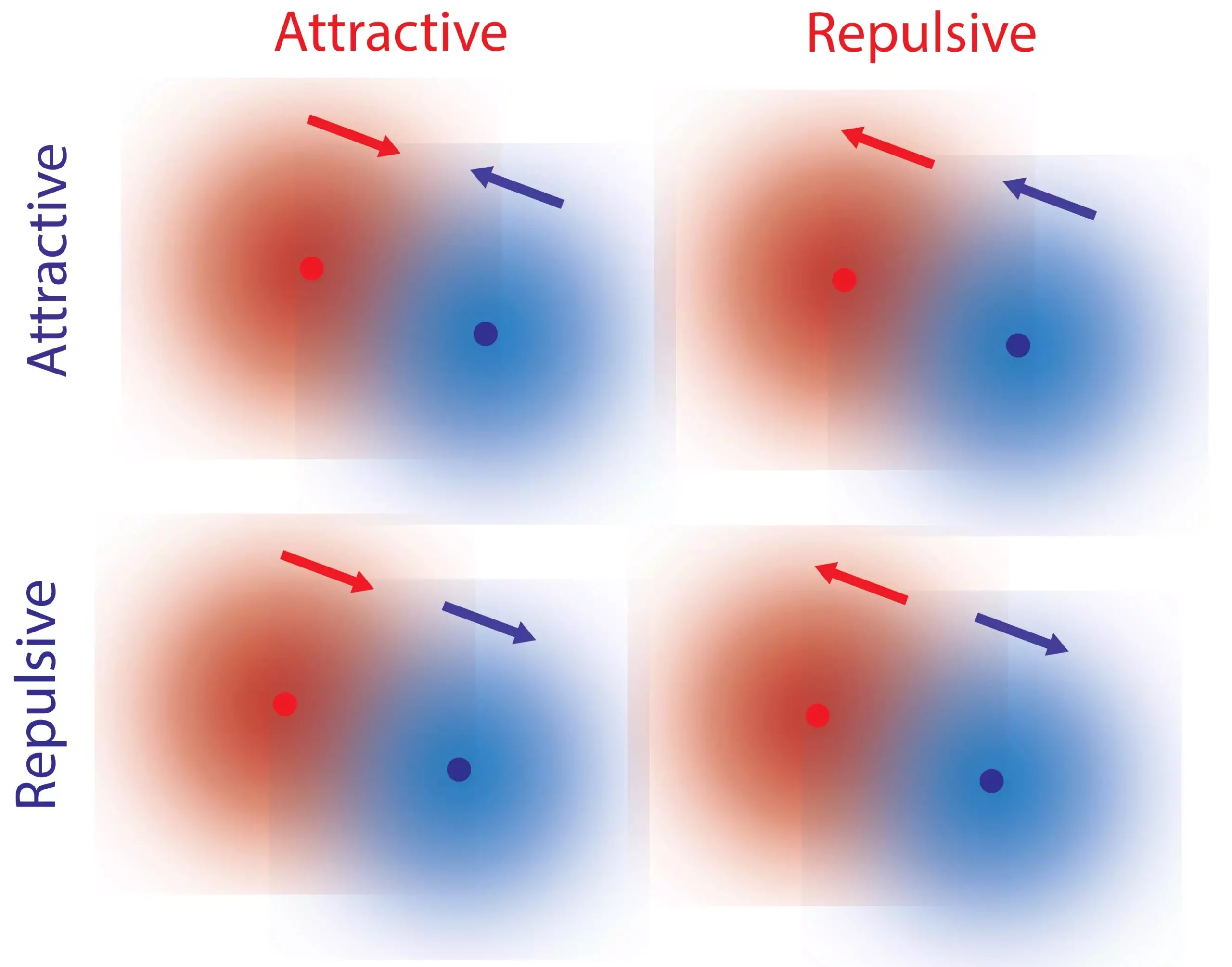
The researchers discovered that the local gradients of reactants and products, resulting from chemical reactions facilitated by catalysts, contribute to non-reciprocal interactions between single molecules. Catalysts, such as enzymes, possess a property known as kinetic asymmetry, which determines their response to concentration gradients.
The “Eureka moment” for the authors came when they realized that this kinetic asymmetry, inherent to catalysts, plays a pivotal role in controlling the direction of molecular response to concentration gradients. Since kinetic asymmetry is an inherent property of enzymes, it has the potential to evolve and adapt. This mechanism provides insights into how molecules are both repelled and attracted to each other, enabling complex interactions and potentially contributing to the evolution of life.
The research conducted by Mandal, Sen, and Astumian expands upon earlier studies in the field of non-reciprocal interactions and “active matter.” Previous work introduced ad hoc forces to generate non-reciprocal interactions, but the current study unveils a fundamental molecular mechanism that naturally gives rise to these interactions at the single-molecule level.
This research not only deepens our understanding of non-reciprocal interactions but also has practical applications. The kinetic asymmetry that determines the directionality of non-reciprocal interactions between catalysts is also crucial for biomolecular machines. It has been incorporated into the design of synthetic molecular motors and pumps, leading to advancements in the field of molecular engineering.
Moreover, the collaboration between Astumian, Sen, and Mandal seeks to unravel the organizational principles behind the loose associations of catalysts, which may have played a role in the formation of the earliest metabolic structures. Understanding kinetic asymmetry could provide valuable insights into the origins and evolution of life from simple molecules.
The discovery of a novel mechanism for non-reciprocal interactions between single molecules opens up new avenues of exploration in the field of molecular dynamics. By revealing the role of catalysts and kinetic asymmetry, this research uncovers the fundamental principles that drive complex behavior at the molecular level. The implications of this study extend far beyond the realm of physics and chemistry, offering potential insights into the origin of life itself. As scientists continue to push the boundaries of knowledge, the understanding of non-reciprocal interactions will undoubtedly transform our understanding of the world around us.

Leave a Reply