Technology advancements often rely on efficient and selective catalysts for various industrial applications. In recent years, researchers have been exploring the conversion of carbon dioxide (CO2) into raw materials using electrocatalysis. While most experiments have involved heterogeneous catalysts, which are in a different phase than the reactants, homogeneous catalysts have been considered to be more efficient. However, until now, there has been a lack of set-ups to test homogeneous catalysts under industrial conditions. In a groundbreaking study, a collaborative team from Ruhr University Bochum, the Fraunhofer Institute for Environmental, Safety and Energy Technology UMSICHT, the Johannes Kepler University Linz, and the Fritz Haber Institute in Berlin has successfully addressed this gap. Their research, outlined in the journal Cell Reports Physical Science, presents an innovative approach that could revolutionize CO2 conversion and pave the way for sustainable resource utilization.
Led by Kevinjeorjios Pellumbi and Professor Ulf-Peter Apfel, the team aimed to establish an efficient solution for CO2 conversion using homogeneous catalysts. Homogeneous catalysts are in the same phase as the reactants, making them highly desirable due to their efficiency and selectivity. The researchers integrated metal complex catalysts into the electrode surface, without chemically bonding them to it. This integration allowed for the efficient conversion of CO2, generating current densities of more than 300 milliamperes per square centimeter. Impressively, the system remained stable for over 100 hours without any signs of decay. These findings open up new possibilities for the use of high-performance and easily variable homogeneous electrocatalysts in various electrochemical processes.
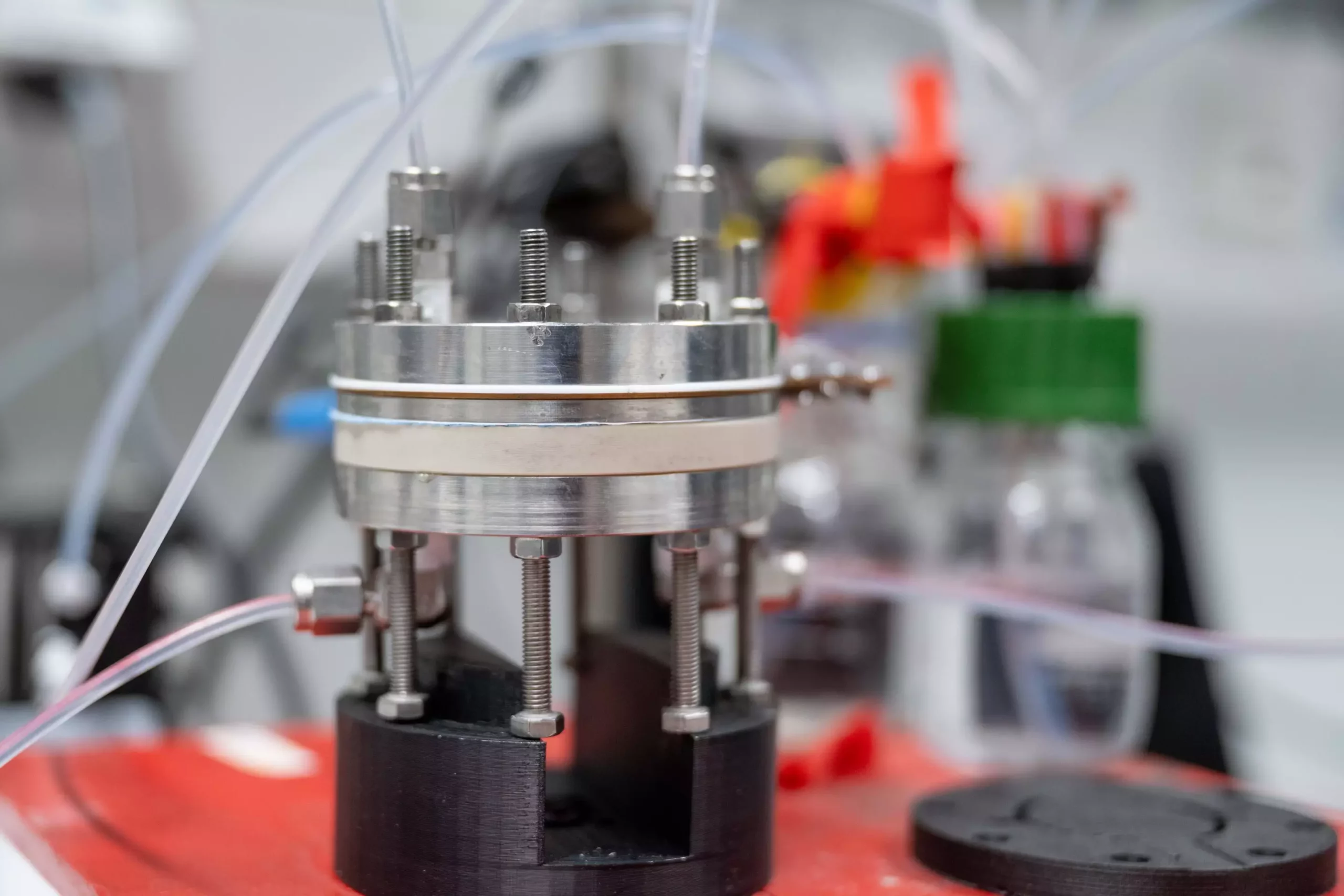
The conversion of CO2 using electrocatalysis involves the supply of electrical energy through a voltage source. This energy is then fed to the reaction system via electrodes, driving the chemical conversions at the electrodes. In the case of homogeneous electrocatalysis, a dissolved metal complex serves as the catalyst. In the experiment, the researchers utilized a gas diffusion electrode, where CO2 flowed past the electrode. The catalysts integrated into the electrode surface facilitated the conversion of CO2 into carbon monoxide, a common starting material in the chemical industry.
The successful integration of homogeneous catalysts into the electrode surface demonstrates their potential for use in electrolysis cells. Unlike previously believed, there is no requirement for a carrier material to chemically couple the catalyst to the electrode surface. Additionally, the researchers found that the electrodes must be able to enable direct gas conversion without solvents. This ensures that the catalyst remains on the electrode surface, preventing it from being leached away. By eliminating the need for a carrier material, this breakthrough allows for easier testing and integration of high-performance and easily customizable homogeneous electrocatalysts in various electrochemical processes.
The study conducted by the collaborative research team represents a significant step forward in the field of CO2 conversion. By demonstrating the viability of homogeneous catalysts and their compatibility with industrial conditions, this research opens up new avenues for sustainable resource transformation. The ability to efficiently convert CO2 into valuable raw materials contributes to the global efforts in combating climate change and reducing greenhouse gas emissions. Furthermore, the integration of high-performance homogeneous electrocatalysts into electrochemical processes could revolutionize various industrial sectors, enabling the use of greener and more efficient technologies.
The development of efficient and selective catalysts is crucial for advancing technology and addressing global challenges. The recent breakthrough in testing homogeneous catalysts for CO2 conversion represents a significant achievement in the field of electrocatalysis. The successful integration of metal complex catalysts into the electrode surface allows for the efficient conversion of CO2, generating promising current densities. By eliminating the need for a carrier material, this breakthrough paves the way for future advancements in electrochemical processes. The research conducted by the collaborative team offers new possibilities for sustainable resource utilization and holds great potential for transforming CO2 into valuable raw materials. With continued research and development, this breakthrough could play a vital role in mitigating climate change and fostering a more sustainable future.

Leave a Reply