Almost three decades ago, a groundbreaking discovery was made by scientists in the field of organic chemistry. A unique class of anticancer molecules was found in a group of marine invertebrates known as bryozoans. These molecules, with their intricate structures made up of oxidized rings and nitrogen atoms, have posed a challenge to researchers globally, who have been attempting to replicate them in a laboratory setting.
The molecules were initially identified in certain species of bryozoans, small aquatic animals that feed by filtering prey from the water. These marine creatures have long been seen as a potential source of new medications, with many of the molecules isolated from them showing promise as anticancer agents. However, the complexity of these molecules has often hindered further development in the field of medicine.
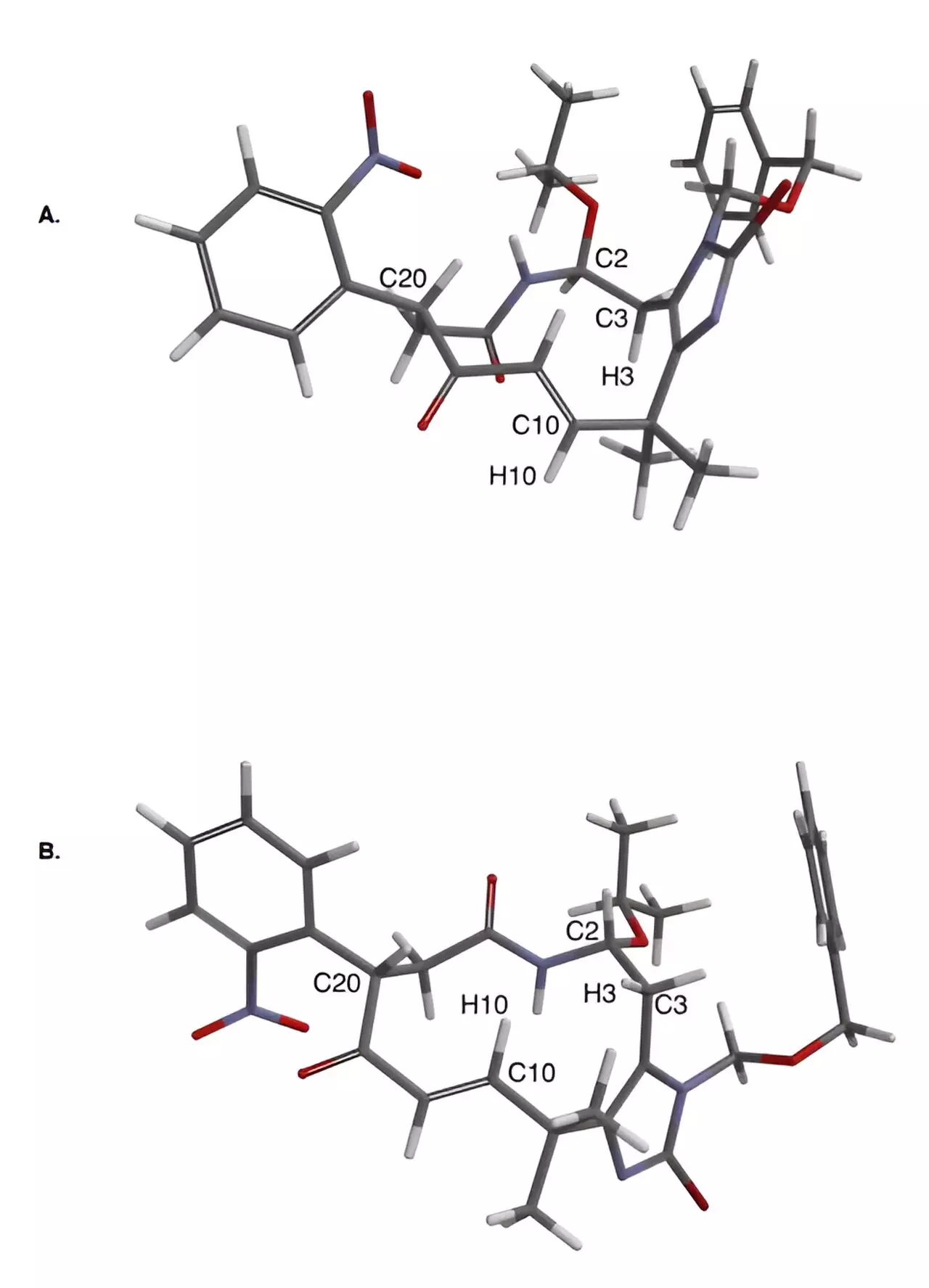
A team of chemists at Yale University, led by Seth Herzon, took on the challenge of synthesizing these elusive compounds. After years of failed attempts, the team successfully created eight of these molecules using a novel approach that integrated inventive chemical strategies with cutting-edge technology in small molecule structure determination. The outcome of their research has been published in the prestigious journal Science.
Herzon and his team employed three crucial strategic elements in their synthesis approach. Firstly, they deferred constructing a reactive heterocyclic ring, known as an indole, until the final stages of the process. This decision was pivotal as the indole ring is notorious for its reactivity and complicating synthesis procedures. Secondly, the researchers utilized oxidative photocyclizations to form essential bonds within the molecules. One of these photocyclizations involved the interaction of a heterocycle with molecular oxygen, a concept originally explored by Yale’s Harry Wasserman in the 1960s. Lastly, the team utilized microcrystal electron diffraction (MicroED) analysis to visualize the intricate structures of the molecules, as traditional methods fell short in this context.
Read More: Anoxic Marine Basins: A Viable Solution for Carbon Sequestration
The successful synthesis of these molecules signifies a breakthrough in the field of synthetic chemistry. These newly created compounds exhibit therapeutic potential, paving the way for further advancements in cancer treatment. Seth Herzon, the lead researcher, expressed his enthusiasm for tackling complex synthetic challenges and highlighted the chemical reactivity of the molecules as particularly daunting.
The work done by the Yale chemists in synthesizing these anticancer molecules opens up new avenues in the realm of medical research. Their innovative approach, combined with perseverance and advanced technology, has led to the creation of compounds with immense therapeutic promise. This achievement not only sheds light on the intricate chemistry of natural compounds but also holds tremendous potential for the development of novel cancer-fighting drugs in the future.

Leave a Reply