Aluminum oxide, commonly referred to as alumina, is a compound of aluminum and oxygen with the chemical formula Al2O3. This remarkable material is widely recognized not just for its insulating properties but also for its role in various industrial applications. Whether serving as an insulator in electronic components, a substrate in catalytic processes, or a resilient ceramic in harsh chemical environments, aluminum oxide showcases versatility that has captured the attention of scientists and engineers alike. A particularly compelling aspect of this material is its structural complexity, which holds significant implications for its functionality in different applications.
The Complex Nature of Surface Structure
Understanding the surface structure of aluminum oxide is crucial for advancing its applications, particularly in catalysis where reactions occur at surfaces. Inside the crystalline structure of alumina, the arrangement of atoms tends to be orderly and well-defined; however, the surface exposes a different architectural reality. For decades, the precise makeup and arrangement of these surface atoms remained elusive. The insulating properties of aluminum oxide posed substantial challenges for experimental research, leading to a half-century of unanswered questions surrounding its surface structure.
New Research Insights from TU Wien and the University of Vienna
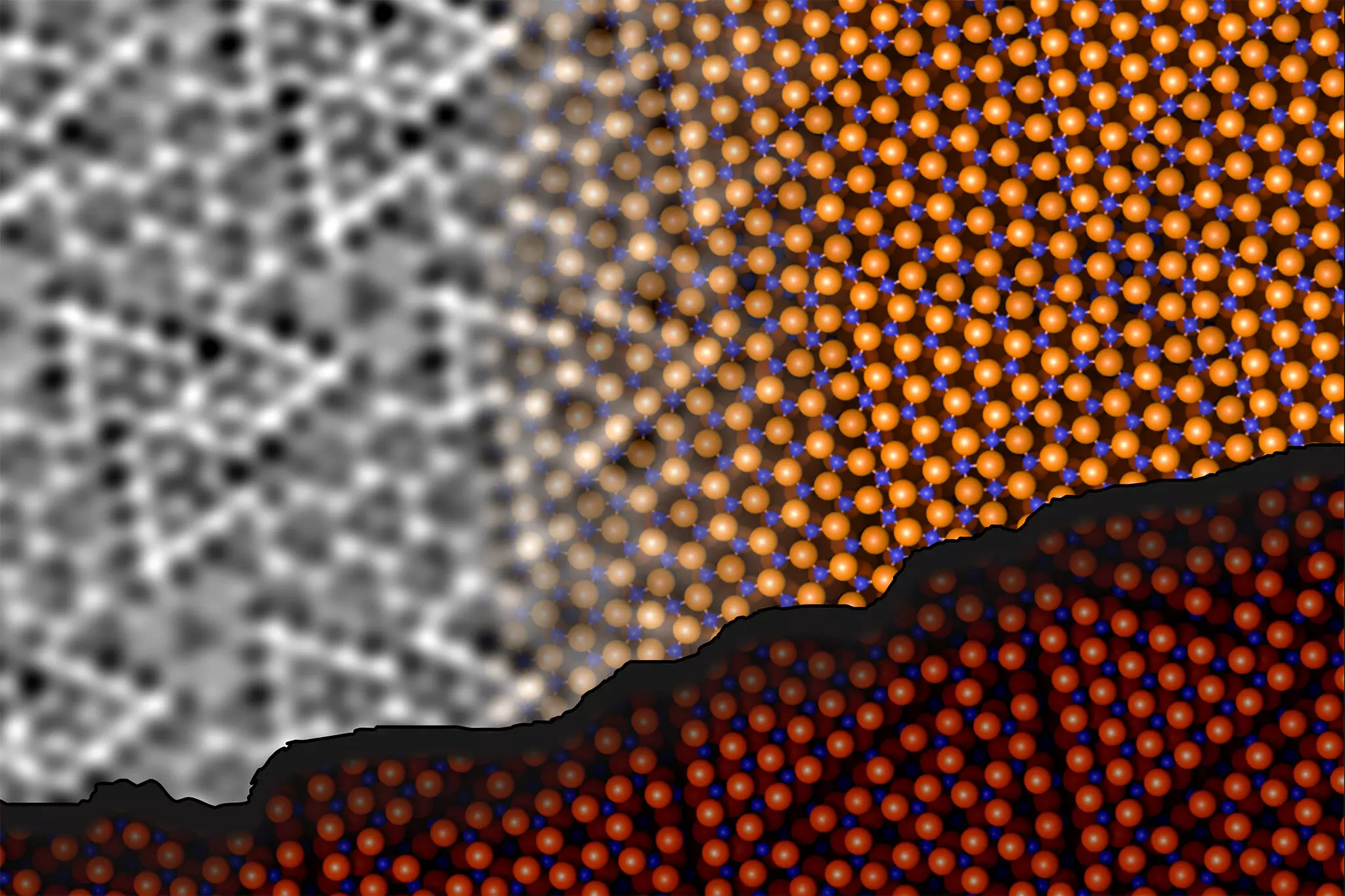
Recent research spearheaded by scholars at TU Wien and the University of Vienna has unveiled groundbreaking insights into the surface structure of aluminum oxide, resolving what was labeled as one of the “three mysteries of surface science” as early as 1997. The findings, published in the journal Science, illustrate the concerted efforts of experts such as Jan Balajka and Ulrike Diebold. Their research utilized noncontact atomic force microscopy (ncAFM), an innovative technique that allows for the imaging of surface structures without direct contact. This technique provides a unique lens through which scientists can view the arrangement of atoms, offering a tantalizing glimpse into the behaviors and interactions that dictate alumina’s properties.
One of the key breakthroughs in this research was the adaptation of ncAFM to enhance chemical sensitivity. Johanna Hütner, who led related experimental efforts, noted that while ncAFM enables the visualization of atomic locations, it typically does not reveal the chemical nature of those atoms. This limitation was overcome through meticulous control of the probe used in measurements. By attaching a single oxygen atom to the tip of the atomic force microscope, researchers could measure the interaction between surface atoms with unprecedented precision. This approach allowed them to effectively differentiate oxygen from aluminum atoms on the surface based on their respective attractions and repulsions.
Unraveling Atomic Relationships and Structural Stability
Through their investigations, the research team discovered a fascinating aspect of the aluminum oxide surface: the aluminum atoms can infiltrate the surface and engage in bonding with oxygen atoms found in deeper layers of the material. This previously unrecognized rearrangement within the initial atomic layers leads to a reduction in energy, thereby promoting structural stability. Contrary to earlier assumptions, the researchers confirmed that the ratio of aluminum to oxygen on the surface remains constant, presenting a challenge to long-standing theories about the atomic interactions at play.
To build their three-dimensional model of the aluminum oxide surface, the researchers harnessed machine learning techniques alongside traditional computational methods. Andrea Conti, a key contributor to this aspect of the project, explained how the complexity of the surface structure complicated the task of establishing a reliable model. The combination of advanced algorithms and meticulous computational exploration ultimately allowed the team to propose a stable structural representation that accurately reflects the unique properties of aluminum oxide.
Broader Implications for Material Science and Future Research
The collaborative nature of this research signifies a monumental step forward in understanding critical materials like aluminum oxide. By demystifying the surface structure of this insulator, the researchers have opened new avenues for exploration not just in catalysis but across a spectrum of industries reliant on material science. The design principles elucidated in this study could lay the groundwork for innovations in various fields, from electronics to sustainable energy solutions. As researchers build on these findings, the implications for enhanced material performance and efficiency are bound to unfold, ultimately driving technological progress in a myriad of applications.
The journey to uncovering the secrets of aluminum oxide has not only solved a long-standing puzzle but has also illuminated the path forward for future advancements in material science and engineering.

Leave a Reply