The concept of using solar energy or other forms of renewable energy to split water and produce hydrogen (H2) on a large scale holds great promise for sustainable energy production. However, many photoelectrochemical water splitting systems that have been proposed so far suffer from inefficiency, instability, or difficulties in implementation. In an effort to address these challenges, researchers at Ulsan National Institute of Science and Technology (UNIST) have developed a novel and efficient photoelectrochemical (PEC) system for green hydrogen production. This article takes a critical look at their research and the implications it holds for the future of sustainable energy.
The researchers at UNIST acknowledge the need for at least 10% solar-to-hydrogen (STH) efficiency in order to develop a viable practical PEC system. Selecting an efficient material is identified as the first criterion for achieving this goal. While previous attempts at photoelectrochemical hydrogen production relied on stable metal oxides as photoelectrode materials, the efficiencies achieved were far below the desired levels. Some researchers have explored the potential of using photovoltaic (PV) grade materials, such as silicon, perovskites, chalcogenides, and III-V material classes. However, these materials can be expensive and unstable when exposed to water, which is a requirement for PEC water splitting cells. Metal-halide perovskites (MHP) are seen as an alternative photoelectrode material due to their unique characteristics of high efficiency, low cost, and tunable bandgap. The challenge lies in addressing the stability issues associated with MHP materials.
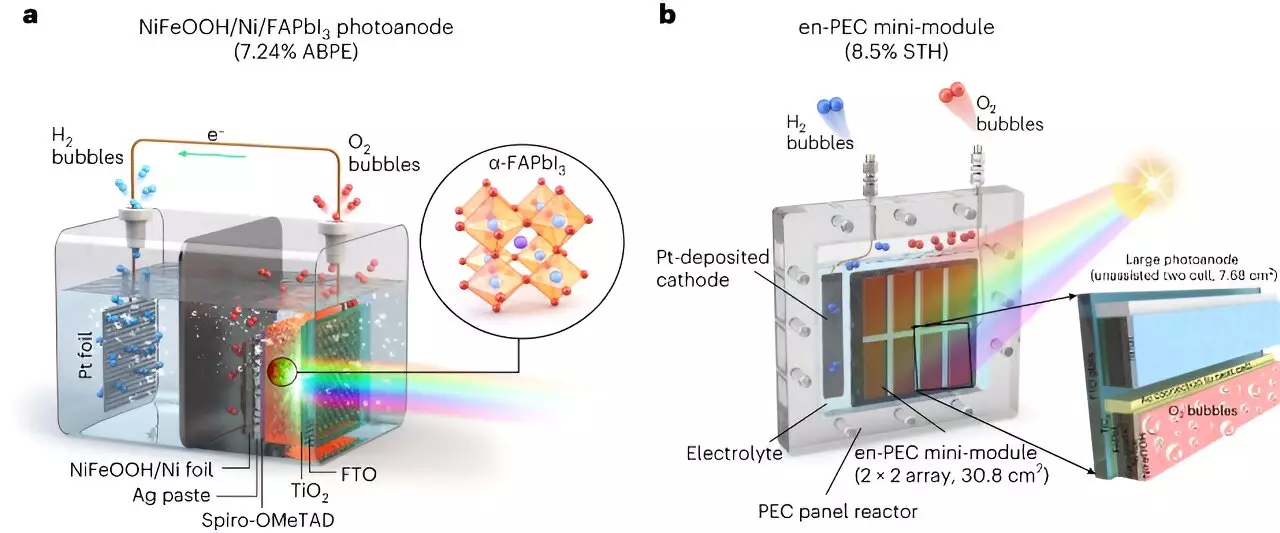
To overcome the stability challenges of MHP materials, the UNIST researchers utilized metal-encapsulation or metal-protection techniques and incorporated the use of UV-stable formamidinium lead triiodide (FAPbI3) perovskite. This allowed them to achieve stability in humid conditions and under UV light. Another challenge faced in practical applications is scalability. The high efficiency achieved in laboratory-scale cells needs to be maintained in large-scale implementations. To address this, the researchers selected FAPbI3 as the most advanced MHP material in terms of efficiency and stability. They encapsulated it with a thick nickel foil deposited with an NiFeOOH catalyst to protect the MHP in water and promote the oxygen evolution reaction required for water splitting.
The researchers initially created a small-scale version of their PEC system, with a photoelectrode smaller than 1cm2. This laboratory-scale system achieved a 9.89% STH efficiency and demonstrated long-term stability. They then scaled up the system using a module-based design, where a 7.68 cm2 device served as the basic mini-module. The mini-module was repeated horizontally and vertically to fabricate a large-size device. Remarkably, the upscaled system only experienced a minimal loss of efficiency and maintained its long-term stability, indicating the high scalability of the design.
The UNIST researchers developed an all-perovskite PEC system composed of an FAPbI3 photoanode protected by a nickel metal foil encapsulation layer and an NiFeOOH catalyst layer. The photoanode was optimized using different metal foils, and the interactions between the catalyst and electrolyte were extensively studied. This photoanode was connected in parallel to another MHP thin film acting as a photovoltaic unit cell in a single reactor. By integrating both components into a single PEC device, the researchers simplified the overall system through a modular design.
The mini-module developed by the UNIST researchers consists of a 4 x 4 array of photoelectrodes and PV unit cells. This integration of multiple components in a single PEC device eliminates the need for additional PV components, reducing system complexity and fabrication costs. Additionally, the researchers demonstrated that their system retains its performance even when deployed on a larger scale. This paves the way for practical applications of PEC technology in outdoor conditions for green hydrogen production. Future research aims to further improve the efficiency and stability of the PEC system by integrating photoelectrodes and selecting more efficient and durable catalysts.
The research conducted by the UNIST team offers promising advancements in the field of photoelectrochemical water splitting for sustainable hydrogen production. By addressing the challenges of efficiency, stability, and scalability, their all-perovskite PEC system demonstrates great potential for practical applications. As we pursue a transition towards a clean and renewable energy future, such innovative technologies play a crucial role in achieving our goals.

Leave a Reply