In recent years, interdisciplinary research has become a cornerstone of scientific advancement, particularly at the intersection of physics and biology. A compelling example of this can be found in the study of protein compartmentalization and its implications for cellular function. Researchers at São Paulo State University (UNESP) in Rio Claro, Brazil, have made significant strides by applying concepts from condensed matter physics to elucidate phenomena in biological systems. Their work provides insights into how cellular dynamics may be governed by principles akin to those seen in the Griffiths phase observed in magnetism, revealing patterns of organization within cellular processes akin to physical systems exhibiting phase separation.
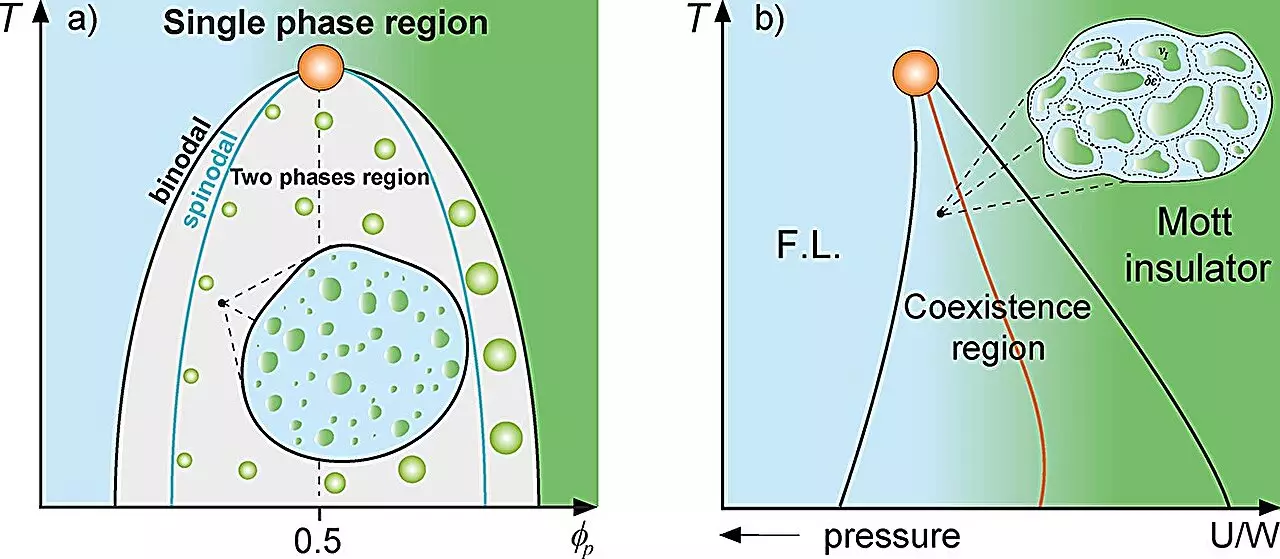
To understand the relevance of a Griffiths-like phase in cellular biology, one must first grasp the foundational principles behind the magnetic Griffiths phase. This concept arises in the context of materials where magnetized and non-magnetized domains coexist, often leading to unusual dynamics that deviate from expected patterns. This analogy holds true for biological systems where sections of protein-rich environments within cells, termed “rare regions,” emerge spontaneously due to local concentrations of proteins undergoing liquid-liquid phase separation. These rare regions can substantially alter the cellular landscape, mirroring how magnetic interactions can lead to different behavioral regimes in physical materials.
Mariano de Souza, the principal investigator of the study and a professor at IGCE-UNESP, along with his Ph.D. candidate Lucas Squillante, focused their research on these protein droplets as analogs to the rare regions found in magnetic systems. Their results suggest that just as magnetized areas can influence the overall dynamics of a material, these protein compartments play a similar role in cellular environments, impacting processes like gene expression and cellular response to stimuli.
Employing an array of thermodynamic models, including the Grüneisen parameter and the Flory-Huggins model, Souza and his team demonstrated that the dynamics within cells slow markedly in proximity to the binodal line—a threshold marking the onset of phase separation. This slowdown mirrors the emergence of Griffiths phases in physical systems, where the dynamics of the material are greatly affected by localized regions of differing properties. Such findings have profound ramifications, suggesting that the biological context of protein dynamics may benefit from a similar theoretical framework as that used in condensed matter physics.
The implications of this slowing down of dynamics are particularly fascinating. It indicates that the fluid properties of protein droplets might be crucial for efficient cellular function, with slower molecular movement potentially allowing for more orderly interactions among proteins. This hypothesis not only enhances our understanding of protein behavior but could also provide frameworks for investigating pathologies associated with protein misfolding and aggregation.
Interestingly, this research also delves into broader existential questions, such as the origins of life. The study posits that the Griffiths-like cellular phase might share commonalities with primordial cellular environments, similar to ideas proposed by the biochemist Aleksandr Oparin. Oparin’s hypothesis suggested that life on Earth began in a “prebiotic soup” of organic compounds. The researchers propose that overloaded protein concentrations might lead to the genesis of coacervates—droplets that encapsulated cellular functions and dynamics, forming the basis for early life forms. By linking these protein dynamics to fundamental questions of biological evolution, the study not only elucidates cellular mechanics but also opens avenues for understanding life’s beginnings.
A particularly thrilling aspect of this research is its potential implications for health and disease. Disorders such as cancer, neurodegenerative diseases, and even viral infections like COVID-19 have been linked to aberrations in protein phase separation. By understanding the sophisticated dynamics of protein droplets through the lens of the Griffiths-like phase, the research team provided essential insights that may pave the way for novel therapeutic strategies. The capacity of liquid-liquid phase separation to influence gene expression and cellular mutation dynamics fundamentally alters our approach to tackling these diseases.
As Minicucci, a co-author of the study, acknowledged, the relationship between phase separation and various diseases is complex. Recognizing that protein droplet formations can have diverse outcomes allows for a more nuanced understanding of cellular processes and the development of targeted therapies.
The work conducted by Souza and his collaborators exemplifies the potential of interdisciplinary research in illuminating complex biological phenomena. By blending principles of condensed matter physics with cellular biology, they have constructed a compelling narrative around protein compartmentalization. This integrative approach not only enhances our comprehension of cellular dynamics but also provides promising pathways for addressing significant health challenges. As the boundaries between scientific disciplines continue to blur, such studies will undoubtedly be at the forefront of future breakthroughs in understanding life’s intricate mechanisms.

Leave a Reply