Recent advancements in material science shed light on the intricate characteristics of high entropy oxides, which play a significant role in modern electronic devices. A groundbreaking study published in the Journal of the American Chemical Society has revealed the profound influence of various synthesis methods on the structural and functional properties of these materials. Notably, high entropy oxides consist of multiple transition metal oxides and exhibit remarkable electrochemical properties, making them promising candidates for future technologies.
Understanding the Study’s Context
Led by Alannah Hallas from the University of British Columbia, the research investigates a specific type of high entropy oxide characterized by a spinel crystal structure formed from a combination of five different transition metal oxides. The study emphasizes the flexibility and variability in the synthesis of these materials. This flexibility translates to numerous possibilities in designing and optimizing high entropy oxides for applications ranging from batteries to catalysts. Hallas’s research indicates that these materials possess significant potential due to their diverse chemical properties and adaptability.
Comparative Analysis of Synthesis Methods
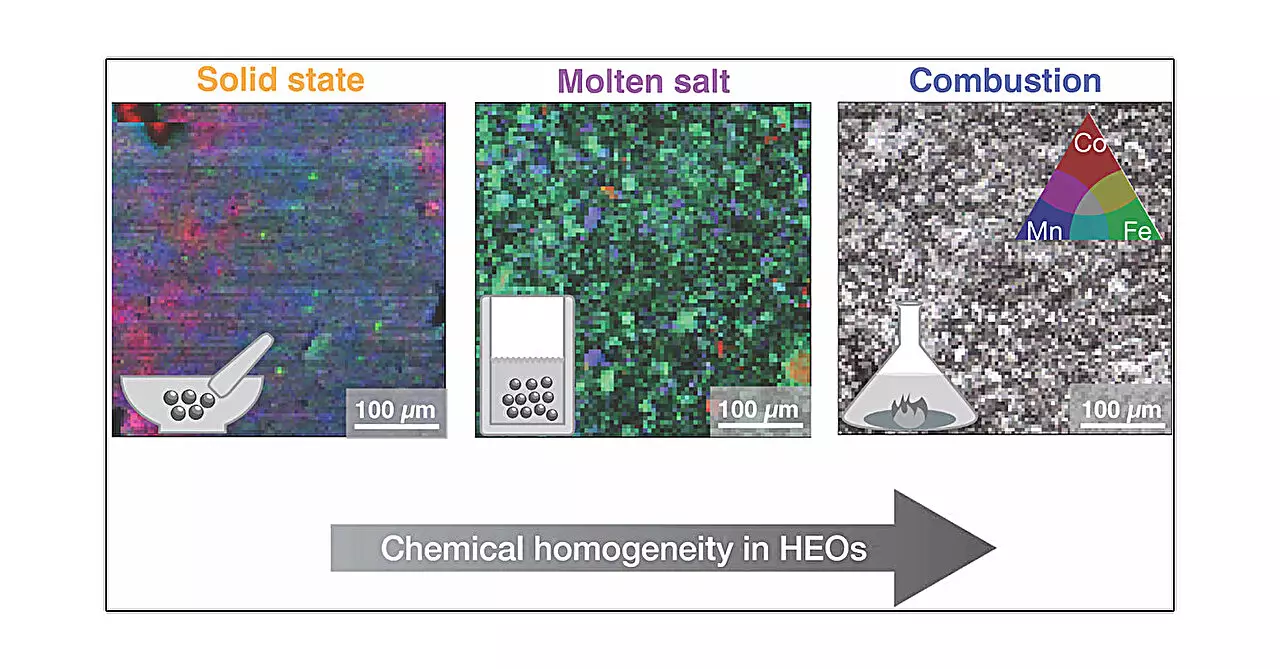
The researchers conducted a series of experiments, employing five distinct synthesis methods: solid-state synthesis, high-pressure synthesis, hydrothermal synthesis, molten salt synthesis, and combustion synthesis. Each method presents unique parameters, such as heating rates, cooling processes, and environmental conditions, leading to diverse outcomes for the same base material. This investigation provides crucial insights into how each synthesis method affects local structures and microstructures, despite findings suggesting that the average structure remains consistent across methods.
Solid-state synthesis involves blending metal oxides and applying heat, akin to traditional baking. In contrast, high-pressure synthesis introduces external pressure, which can yield significant variations in material formation. The hydrothermal method is designed to mimic natural crystalline processes, utilizing pressurized water to enhance crystal growth, while the molten salt method uses a heated, viscous liquid to precipitate crystal structures as it cools. The combustion synthesis method is intriguing, where metal salts dissolved in water create a gel that ignites, facilitating rapid material production.
One of the critical findings of this study is the realization that although the average structural attributes of the high entropy oxides might appear unchanged among the different synthesis methods, their local configurations can vary significantly. According to Mario Ulises González-Rivas, the study’s lead author, combustion synthesis yielded the most homogeneous samples. This observation emphasizes the necessity of understanding microstructural impacts when tailoring materials for specific applications, particularly in energy systems where performance can be influenced by subtle structural nuances.
As summarized by González-Rivas, the implications of this research extend beyond laboratory findings. The structural variations induced by different synthesis processes highlight a crucial dimension for optimizing high entropy oxides for commercial use. Energy challenges faced globally could benefit from the technological application of these materials, thus making it imperative to consider these optimization pathways in practical settings.
This study is the result of a collaborative effort encompassing the resources and expertise from various institutions, including researchers from the University of Saskatchewan and the Max Planck Institute for Solid State Research. Such interdisciplinary collaborations underline the importance of diverse perspectives in scientific inquiries and can lead to innovations that push the boundaries of material science.
Looking ahead, this research opens avenues for further investigations into high entropy oxides. Future studies can delve deeper into how these synthesis variations affect other properties and explore novel applications that could capitalize on the unique attributes of these materials. Additionally, researchers are encouraged to explore the commercial viability and scalability of these synthesis methods, aiming to transition theoretical discoveries into real-world applications.
The study of high entropy oxides showcases the critical role that synthesis methods have in determining their structural and functional properties. As technology continues to advance and demand innovative materials, understanding and harnessing the various methods of synthesis will play a vital role. This research not only contributes to the foundational knowledge of material science but also holds the potential for significant technological advancements in the field globally.

Leave a Reply