In the quest to combat climate change, innovative solutions are pivotal for reducing carbon footprints. One groundbreaking approach is artificial photosynthesis—a bio-inspired technology that mimics natural processes to convert carbon dioxide (CO2) and water into useful energy forms. Researchers at the University of Michigan have developed an advanced artificial photosynthesis system that efficiently chains carbon atoms, creating hydrocarbons with remarkable performance. This new methodology provides a promising pathway toward producing sustainable fuels and reducing reliance on fossil fuels.
The University of Michigan’s system distinguishes itself by producing ethylene—a basic hydrocarbon primarily utilized in the plastics industry. This working model showcases an impressive efficiency and stability, boasting performance metrics five to six times superior to existing solutions for reducing CO2 to ethylene. Professor Zetian Mi, a pivotal figure in this innovative project, notes that, despite ethylene being one of the most produced organic compounds globally, its conventional production method generates significant CO2 emissions, typically derived from oil and gas under extreme temperatures and pressures.
The ability to redirect CO2 emissions into something useful, such as synthetically produced plastics, could drastically curtail greenhouse gas emissions. The prospect of making ethylene from captured CO2 exemplifies how scientific advancements can provide viable solutions for environmental challenges.
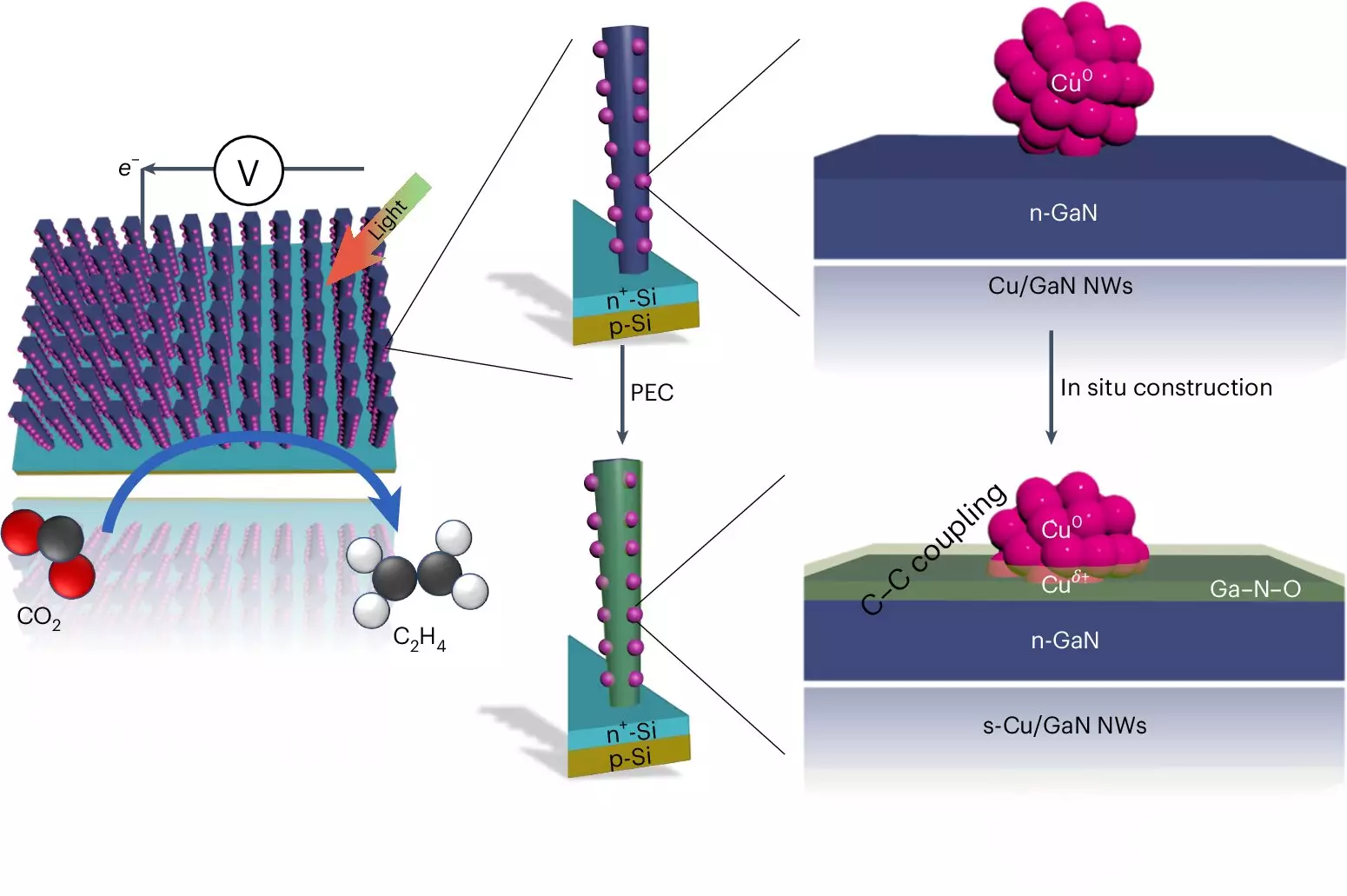
At the core of this artificial photosynthesis system is a unique device that employs advanced semiconductor technology. By integrating gallium nitride nanowires—extremely slender structures measuring just 50 nanometers in diameter—with a silicon substrate, researchers harness the power of sunlight to drive the chemical reactions necessary for ethylene production. The process begins as the device absorbs sunlight, releasing electrons that enable water splitting, yielding both hydrogen and oxygen.
Interestingly, it is the copper clusters that facilitate the critical conversion of CO2 into hydrocarbons. With approximately 30 atoms, these clusters capture the hydrogen generated and interact with carbon atoms, forming carbon monoxide as a preliminary step to creating ethylene. This interaction occurs at the interface of the copper and gallium nitride oxide, stripping away excess oxygen and replacing it with hydrogen, ultimately facilitating the bonding of carbon atoms.
One of the most significant advances of the Michigan team’s device is its remarkable longevity and sustained effectiveness. Surpassing its silver and copper-based counterparts, which typically degrade within a few hours, this innovative device has demonstrated operational viability for over 116 hours in a single run, even reaching 3,000 hours in similar setups. The synergy between gallium nitride and the oxygen produced during water splitting enhances the catalyst effectiveness, further establishing a self-healing capability that ensures extended operational life and productivity.
In terms of production rate, this pioneering technology achieves ethylene output over four times that of the closest competitive systems. This efficiency demonstrates a monumental step forward in the quest for scalable, sustainable fuel production methods capable of transforming energy markets.
The implications of the Michigan study extend far beyond the production of ethylene. Researchers aim to explore the synthesis of longer-chain hydrocarbons, such as propanol and other liquid biofuels, which would facilitate compatibility with existing transportation infrastructures. By generating sustainable liquid fuels, researchers hope to enable the transition towards a greener economy without sacrificing technological utility.
As the team prepares for further experimentation, the focus will also include assessing the upper limits of durability for these advanced systems. Bridging the gap between current fossil fuel processes and sustainable alternatives is no small task; however, the ongoing research holds the potential to transform how we perceive energy independence and environmental stewardship.
Artificial photosynthesis technology, as demonstrated by the research team at the University of Michigan, presents an exciting frontier in energy production. By effectively converting CO2 emissions into valuable hydrocarbons, the potential for sustainable fuels becomes more tangible. As the research progresses, these innovative approaches not only represent a crucial step in addressing climate change but also pave the way for a clean and sustainable future. Harnessing light to tackle our reliance on fossil fuels symbolizes the essential union of science, technology, and environmental responsibility in the 21st century.

Leave a Reply