Transcranial focused ultrasound (tFUS) has emerged as a promising non-invasive approach to stimulate targeted brain areas using high-frequency sound waves. This method offers great potential, particularly for treating challenging neurological conditions like drug-resistant epilepsy and tremors, which have proven difficult to manage with conventional treatments. A team from Sungkyunkwan University (SKKU), the Institute for Basic Science (IBS), and the Korea Institute of Science and Technology has made significant strides in this domain by developing a groundbreaking sensor intended to enhance the efficacy of tFUS on patients.
Unprecedented Shaping and Adhesion
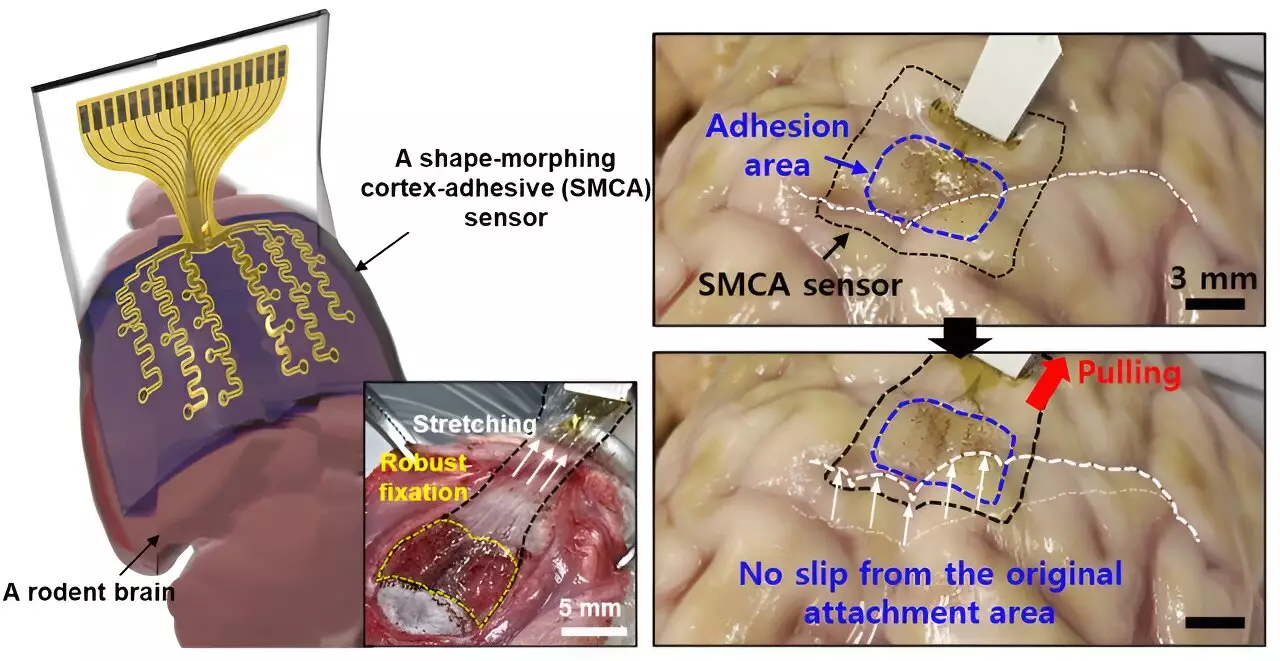
The innovative sensor was recently detailed in a study published in Nature Electronics. One of the standout features of this new technology is its ability to adapt its shape, allowing it to conform closely to the brain’s intricate cortical surfaces. This capability addresses previous limitations in sensor technology that hindered accurate neural signal recording. Donghee Son, the supervising author of the study, highlighted that prior brain sensors struggled due to the rigid nature of their designs, preventing them from adequately covering the complex folds and ridges of the brain’s surface.
“Prior inventions were not designed to match the dynamic characteristics of the brain’s surface,” Son stated. Past attempts to create brain sensors led to significant inaccuracies in signal measurements, especially in regions where the brain curvature was more pronounced. Such limitations posed challenges in mapping brain lesions, which is critical for diagnosing and developing treatment strategies.
Enhancing Long-term Measurement Success
Building on the groundwork laid by previous researchers, particularly Professors John A. Rogers and Dae-Hyeong Kim, who created a thin sensor with improved surface measurements, Son’s team took it a step further. Their new sensor can adhere firmly to a variety of brain curvature configurations, allowing for prolonged and reliable measurement of brain signals. This crucial advancement could enable clinicians to gather more accurate information from specific brain regions over sustained periods.
The device, referred to as ECoG, showcases an impressive mechanism for adhering to neural tissue. With no voids created in the attachment, this minimizes unwanted noise and movement that can distort readings from external mechanical sources. “The capacity to maintain strong contact with brain tissue is paramount for effectively treating conditions like epilepsy,” Son remarked, emphasizing the critical role of consistent data in devising treatments based on real-time brain activity.
Shaping Personalized Treatments for Epilepsy
Recent years have witnessed extensive research into personalized treatment approaches for epilepsy and other neurological disorders, driven by the understanding that the effectiveness of therapies can significantly vary between individuals. The ability to measure brain activity in real-time is essential for tailoring treatments to meet varying patient needs. Nevertheless, conventional sensors fell short, facing interference from the vibrations induced by ultrasound stimulation and generating substantial noise.
Son’s sensor surmounts this challenge, drastically cutting down the noise levels during real-time monitoring. This capability carries immense implications for utilizing low-intensity focused ultrasound (LIFU) techniques, allowing clinicians to customize interventions for epilepsy by effectively mapping brain waves while concurrently delivering ultrasound stimulation.
A distinct characteristic of the sensor’s architecture lies in its three-layer structure. Firstly, a hydrogel layer forms strong bonding with the brain tissue, physically and chemically. Secondly, a self-healing polymer layer morphs to the underlying surface features, ensuring optimal contact over time. Finally, the outermost layer, which comprises stretchable and ultrathin materials integrated with gold electrodes, maintains efficient communication during the sensor’s operational phases.
When placed on the brain’s surface, the hydrophilic properties of the gel initiate a rapid and robust attachment to brain tissue, enabling the self-healing polymer to adapt accordingly—a process that significantly enhances its performance.
The breadth of possibilities stemming from this innovative sensor approach extends beyond the treatment of epilepsy. According to Son, the sensor technology could transform the diagnostics and treatment landscape for various neurological disorders. “The dual advantage of tissue adhesion and shape adaptation will revolutionize how we approach brain therapies,” he predicts, underscoring the significance of the dual-function technology in making strides toward high-resolution brain signal mapping.
Looking Forward: Future Developments and Scalability
Initial tests conducted on awake rodents exhibited encouraging results, demonstrating the sensor’s capacity to accurately monitor brain waves and manage seizure activities. With aspirations to scale up the sensor into a high-density array while transitioning to clinical trials, the team plans to enhance the sensor further by increasing the number of electrode channels—improving the granularity of brain signal analysis.
The implications of this research might not only reshape the treatment landscape for epilepsy but could also contribute to significant advancements in prosthetic technology. As researchers work towards refining this promising technology, the potential for improved patient outcomes appears increasingly within reach, heralding a new chapter in the management of neurological disorders.

Leave a Reply