The quest for efficient and cost-effective electrocatalysts in the field of energy conversion has seen exciting advancements recently. A groundbreaking study has revealed how the incorporation of erbium, a rare earth element, into cobalt oxide can significantly uplift the performance of catalysts used in oxygen evolution reactions (OER), especially in acidic conditions. This research, highlighted in ACS Catalysis as an Editors’ Choice, not only paves the way for an alternative to pricier noble metals but also offers a glimpse into sustainable energy solutions.
The Challenge of Traditional Catalysts
Oxygen evolution reactions are pivotal in applications such as water splitting, which is essential for efficient energy conversion and storage. Traditional catalysts, primarily based on cobalt oxides, have showcased moderate success in producing oxygen from water. The limitations stem from their inherent spinel crystal structure, which while stable, can hinder optimal catalytic activity. Researchers have long sought methods to enhance the performance of these catalysts, particularly by finding ways to improve their efficiency and durability under acidic conditions—an area traditionally dominated by costly iridium-based catalysts.
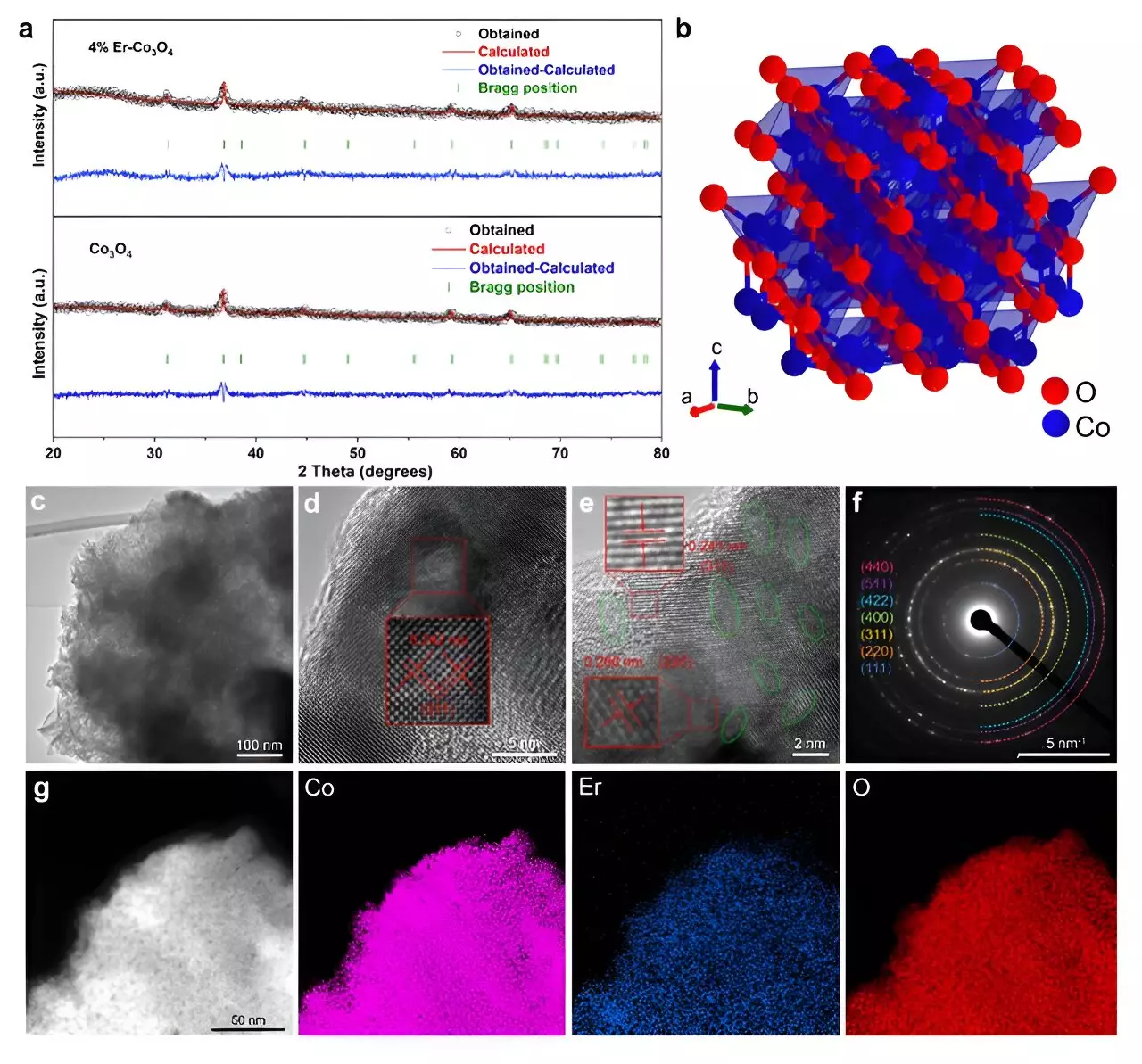
The recent study led by a group of researchers at Tohoku University achieved a significant breakthrough by doping cobalt oxide (Co3O4) with a small percentage of erbium (Er). According to Tianyi Wang, a co-author of the study, this innovative approach resulted in a doped catalyst that showcased an impressive overpotential of 321 mV at a current density of 10 mA cm² and maintained its stability for over 250 hours. These metrics position the modified Co3O4 catalyst as a noteworthy competitor, even against established iridium catalysts, which are known for their superior efficiency yet high costs.
What sets this research apart is the fundamental insight gained on the catalytic process itself. Employing advanced microkinetic modeling alongside density functional theory (DFT) calculations, the team elucidated how erbium integration fostered the formation of active sites within the catalyst’s crystal lattice. This shift dramatically increased the presence of Co3+, crucial for catalyzing the OER.
The research reveals that the increased Co3+/Co2+ ion ratio, influenced by the presence of erbium, plays a pivotal role in creating oxygen vacancies crucial for the OER. By fostering these vacancies, the catalyst effectively increases its activity. The analogy drawn by Hao Li, another co-author, aptly describes the situation: “Imagine the catalyst like a road where Er doping introduces extra lanes.” These “lanes” symbolize the oxygen intermediates—molecules that play a vital role in driving the reaction forward.
In situ Raman spectroscopy further validated the findings by pinpointing the locations of the oxygen vacancies within Co3O4’s octahedral sites. This strategic positioning facilitated the development of important intermediate species that are essential for the OER. With more active Co3+ sites available for anchoring these intermediates, catalytic efficiency reached new heights.
A Sustainable Path Forward
The promising results from this study showcase not only the viability of erbium incorporation in cobalt oxide but also signal a transformative approach to designing future catalysts. The researchers emphasize the importance of balancing electronic properties with structural optimization to push the boundaries of what is achievable with non-noble metals.
As the global community pushes toward renewable energy solutions and sustainable practices, this innovation provides a viable pathway to developing high-performance, cost-effective electrocatalysts. Future research will likely expand on the incorporation of other non-precious metals, further diversifying the arsenal of materials available for advancing energy conversion technologies.
The introduction of erbium into cobalt oxide offers an exciting glimpse into the future of catalysis for energy applications. As researchers continue to unravel the complexities of these interactions, we approach a new era where cost-effective, high-performance catalysts may reshape the landscape of energy conversion and contribute to a more sustainable future.

Leave a Reply