Recent advancements in the field of organometallic chemistry have illuminated new pathways for drug discovery, particularly concerning the functionality of cobalt(III)-based metal complexes. This innovative research spearheaded by Professor Jaeheung Cho and his team at UNIST pivots on the intricate dynamics of nitrile activation, a reaction critical for synthesizing therapeutic compounds. The nuances of cobalt’s reactivities, particularly rooted in its spin states, have opened doors to novel biochemical applications.
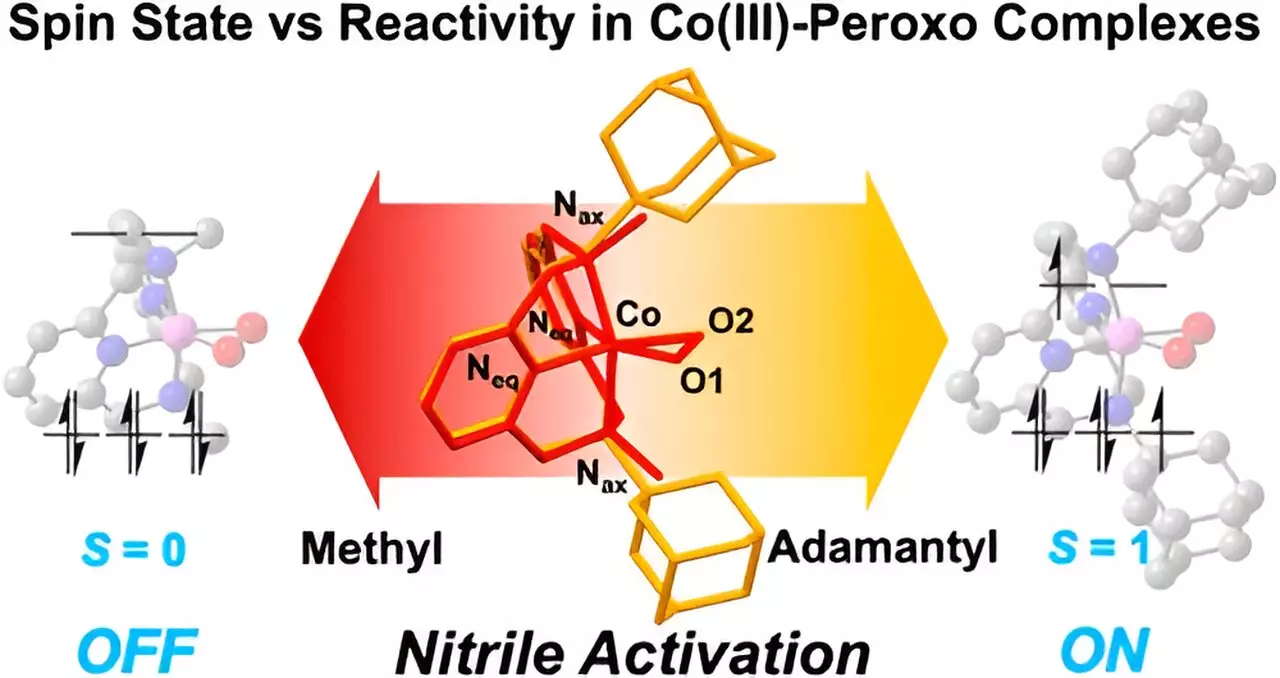
A pivotal insight from Cho’s research is the pronounced effect that metal spin states exert on reaction kinetics. By elucidating the mechanisms involved in the interaction between cobalt(III) complexes and nitrile substances, the study reveals that even minute alterations in the metal’s electronic environment can lead to substantial variations in reactivity. This finding suggests that the design of organometallic compounds can be tailored, making it possible to enhance or inhibit specific chemical reactions based on spin state manipulation.
To dissect the complexities of nitrile and cobalt interactions, the researchers utilized a sophisticated structure known as the “Macrocyclic Pyridinophane System.” This strategically designed framework allowed for the manipulation of cobalt complexes’ architectures, thereby facilitating a controlled study of their behavior toward nitriles. The results were striking; incorporation of larger functional groups, such as adamantyl, led to a marked increase in nitrile activation efficiency, while the presence of smaller groups, like methyl, rendered the complexes inert. This discrepancy indicates that the size of substituents directly correlates with alterations in spin states, thereby influencing the entire reactivity profile.
Nitriles are integral to various pharmaceuticals and agrochemicals, yet their inherent reactivity challenges have limited their broader applications. In this exploration, Cho’s team demonstrated that cobalt(III)-peroxo species could effectively engage with nitriles at ambient temperatures, producing compounds with potential anticancer properties. This finding not only amplifies the scope of cobalt-based chemistry but also underscores the potential of such metal complexes in the pharmaceutical arena, offering a pathway to developing new anticancer therapies.
As the pharmaceutical community seeks innovative solutions to combat cancer, understanding the intricate behaviors of cobalt-based complexes presents an exciting avenue for future exploration. This research heralds a new chapter in drug development, where fine-tuning metal spin states and structural configurations can yield advanced biomimetic compounds. As the field continues to evolve, the significance of metal complexes in medicinal chemistry cannot be overstated; their versatility and the deeply rooted principles highlighted in Cho’s study may pave the way for groundbreaking advancements in targeted therapeutic interventions. The future may hold a plethora of cobalt-based compounds designed specifically for the medical challenges of tomorrow.

Leave a Reply