The quest for sustainable chemical synthesis is a vital area of research in modern chemistry, particularly in the context of drug development and material science. Among the many compounds studied, trisubstituted Z-alkenes stand out for their integral role in the formation of biologically active molecules. These alkenes serve not only as essential building blocks for numerous pharmacological agents but also as substrates in stereospecific reactions that construct complex sp3-hybridized molecular frameworks. However, the challenge of effectively synthesizing Z-isomers—often less thermodynamically stable than their E counterparts—has limited their availability in lab settings.
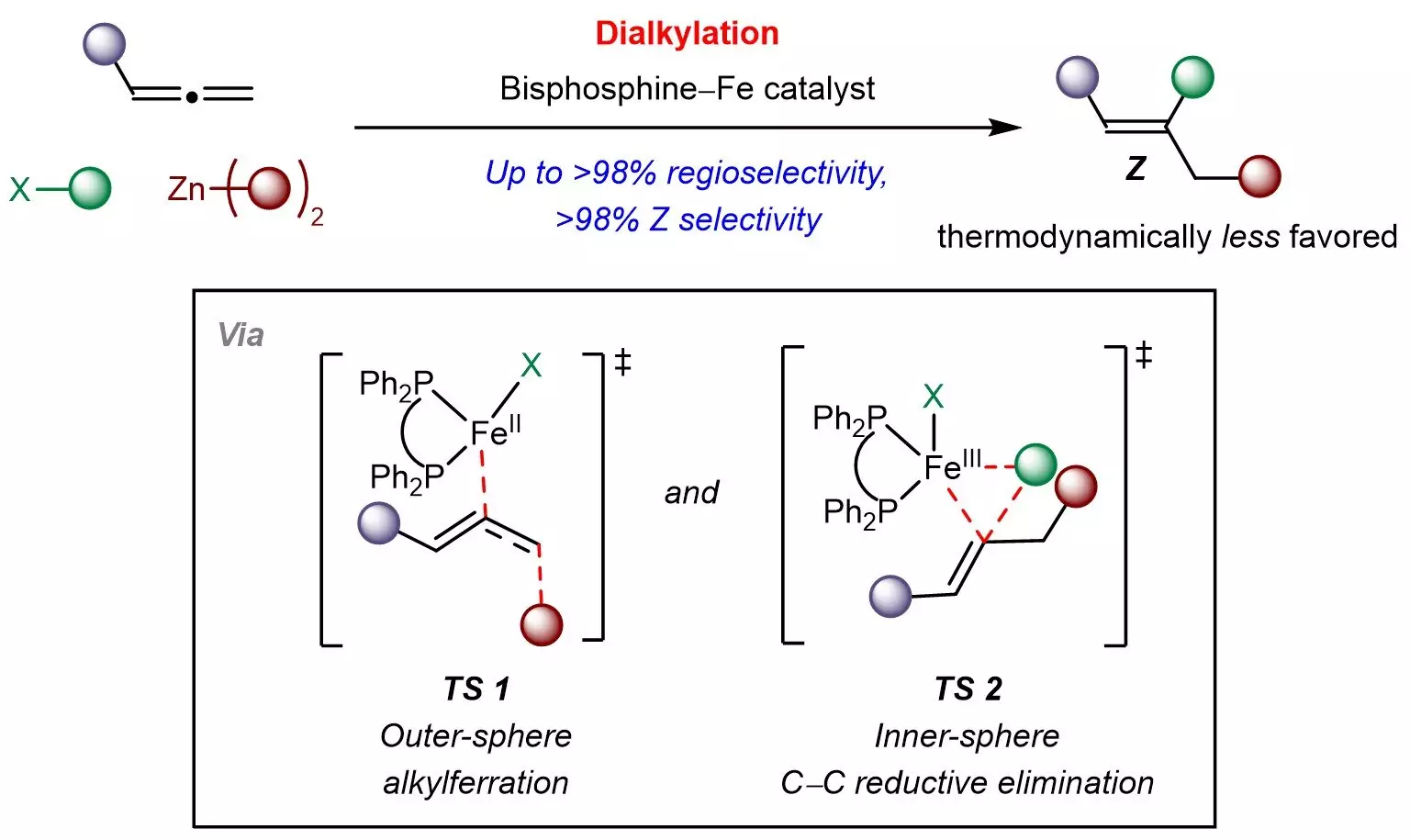
Researchers at the National University of Singapore (NUS) have recently proposed a compelling solution to this dilemma, unveiling an iron-catalyzed method that facilitates the sustainable synthesis of trisubstituted Z-alkenes. The new approach employs an inexpensive and environmentally benign bisphosphine-iron catalyst to integrate allenes with various alkyl groups sourced from readily available organohalides and organozinc compounds. This multicomponent strategy not only enables the effective incorporation of different aliphatic chains but also ensures high selectivity for the Z-isomers. By intelligently navigating the thermodynamic landscape, the team successfully established a kinetically controlled process, which is crucial for yielding the desired product configurations.
Iron’s prominence as a non-toxic and widely abundant transition metal enhances the method’s appeal, aligning well with the principles of green chemistry that advocate for sustainable practices. The affordability of the iron catalyst signifies potential large-scale applications, making this methodology a promising alternative in the realm of synthetic organic chemistry. Economically speaking, the removal of precious metals, which are often favored in catalysis, places this research on a trajectory toward more accessible and scalable chemical production.
What makes this research particularly noteworthy is its application potential. The NUS team demonstrated their technique in synthesizing a glucosylceramide synthase inhibitor, where the Z-configuration was instrumental for its biological activity. This step not only elucidates the practical utility of their method but also highlights its significance in supporting the pharmaceutical industry in the development of potent inhibitors and therapeutic agents.
In addition to the practical applications, the researchers have gained insights into the underlying mechanisms of their method. They suggest a novel reaction pathway that involves radical-mediated alkylferration, followed by the formation of carbon-carbon bonds. Such revelations could pave the way for designing additional kinetically controlled reactions involving allenes, thus expanding the toolkit for synthetic chemists.
Overall, this iron-catalyzed synthesis of trisubstituted Z-alkenes marks a significant contribution to sustainable chemistry, bridging a gap in the accessibility of these crucial compounds. Looking ahead, the research team intends to explore other multicomponent transformations, enhancing the conversion of innate raw materials into valuable chemical entities. This advancement not only promises to foster innovation in chemical research but could also contribute to the development of new strategies for addressing the challenges of drug discovery and industrial applications.

Leave a Reply