Water pollution is a pressing global concern, especially in the context of heavy metals such as cadmium and lead. These toxic metals can infiltrate drinking water supplies, presenting serious health risks to humans and ecosystems alike. The challenge lies not only in the contamination process but also in finding effective and sustainable methods for removing these harmful substances from water bodies. Traditional filtration systems, while somewhat effective, often struggle with energy consumption and the rapid clogging of filtration membranes, necessitating frequent replacements and maintenance.
In response to the shortcomings of conventional techniques, scientists have begun to explore alternative approaches, particularly by looking at molecules derived from plants. Plants naturally produce polysaccharides – complex carbohydrates consisting of long chains of sugar molecules – that serve as a defense mechanism against metal uptake. Recent research has illustrated that these polysaccharides could effectively bind and trap metal ions, making them potent candidates for water purification efforts. However, this natural affinity often comes with its limitations, including issues with solubility and stability in aqueous environments.
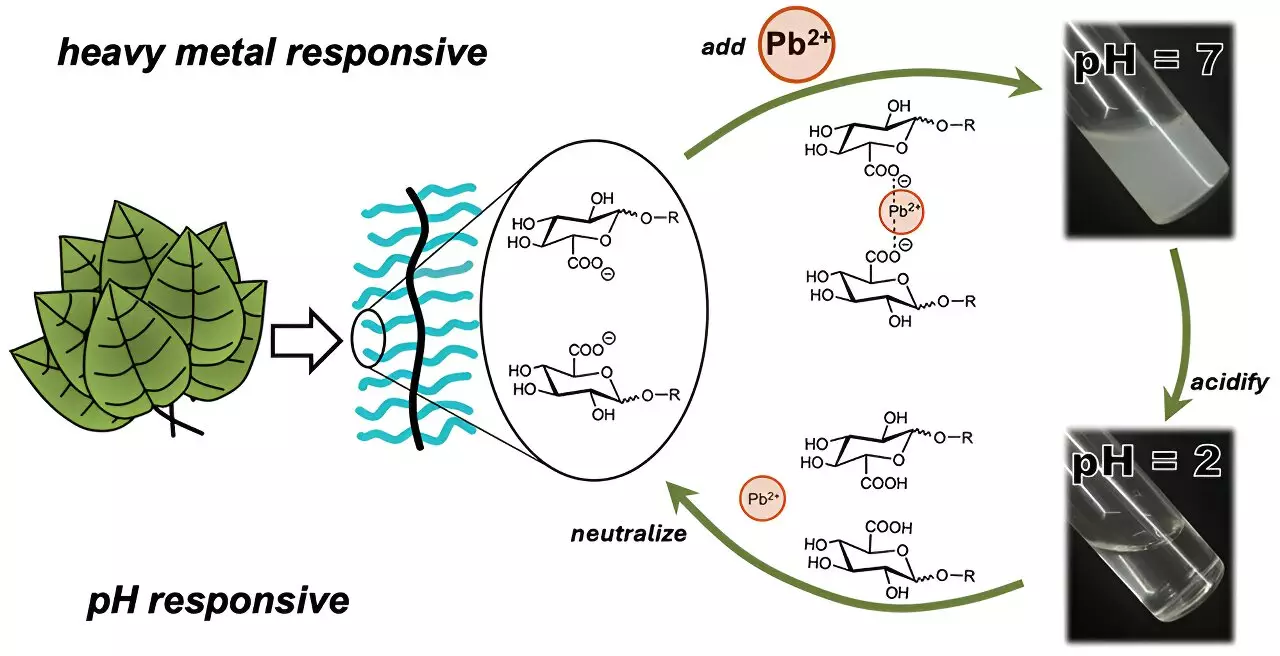
In light of these challenges, a research team led by Cassandra Callmann at the University of Texas at Austin has developed a novel approach utilizing sugar-like polymers designed specifically for the purpose of heavy metal remediation. This innovative material shows promise in its ability to selectively attract and remove harmful metals from water while overcoming solubility issues that have plagued many previous studies. Callmann’s team focused on creating a polymer that blends an insoluble backbone with functional carbohydrate “charms” that can enhance the polymer’s metal-binding capabilities.
Emerging from this research is a unique polymer engineered to form insoluble aggregates that effectively trap ionic heavy metals. What sets this polymer apart is its specific design, which allows the carbohydrate segments dangling from the backbone to interact with metal ions. This adaptable structure comprises a water-insoluble core that does not dissolve in water, while the sugar-like side chains can be tailored to optimize the polymer’s binding efficacy for various heavy metals.
Demonstrating Efficacy in Real-World Conditions
Initial laboratory tests demonstrated the effectiveness of this polymer in binding cadmium ions, where it formed visible clumps in a matter of minutes. The most successful carbohydrate component contained a carboxylic acid group that facilitated strong bonding with cadmium. Importantly, these clumped bindings could be filtered out easily, marking a significant advancement toward practical applications.
Further tests involved spiking a sample of natural Colorado River water with both cadmium and lead. Despite the presence of competing ions like calcium, sodium, and magnesium, this polymer managed to extract substantial quantities of the target heavy metals, retrieving approximately 20% of cadmium and about 45% of lead over a 24-hour testing period. These promising results suggest not just efficacy but also selectivity, which is crucial in real-world applications, where multiple contaminants often coexist.
This development is more than a mere incremental improvement; it symbolizes a fundamental shift toward more efficient, environmentally friendly solutions in the field of water purification. The researchers are optimistic that the polymer’s recyclability – demonstrated through its ability to consistently trap and release metal ions without degradation – presents sustainable prospects for practical use.
As the world grapples with the implications of polluted water supplies, finding scalable and cost-effective solutions is more important than ever. This cutting-edge polymer technology represents a significant leap forward, offering not only an innovative strategy for tackling heavy metal pollution but also paving the way for enhanced environmental stewardship. Future research and development are likely to refine this technology further, expanding its applications and effectiveness, thereby contributing to the quest for cleaner water globally.

Leave a Reply