Water pollution remains a significant global concern, particularly with the proliferation of pharmaceutical contaminants in waterways. Recent research by scientists at Carnegie Mellon University introduces a compelling solution: an environmentally friendly process that utilizes a TAML catalyst alongside hydrogen peroxide to degrade antibiotics and other pharmaceuticals found in municipal and natural water sources. This breakthrough highlights not only the pressing issue of micropollutants but also the potential for innovative, sustainable methods to address this environmental challenge.
With more than 4,000 prescription medications utilized for both human and animal health, it is no surprise that many end up in the environment. The U.S. Geological Survey has documented the presence of pharmaceuticals in natural water bodies, attributing this phenomenon to improper disposal methods such as flushing expired or unused drugs down the toilet. Conventional wastewater treatment facilities often fail to completely filter out these micropollutants, leading to contamination of rivers, lakes, and groundwater sources. The implications of this pollution can be profound; long-term exposure to pharmaceuticals negatively impacts wildlife, disrupting ecosystems and affecting species from fish to birds.
Despite the evident urgency for effective solutions, many advanced treatments such as ozonation and activated carbon filtration are prohibitively expensive, restricting access to wealthier urban areas. This creates an urgent need for affordable, efficient methods capable of addressing micropollutants across various settings, including rural and underserved regions.
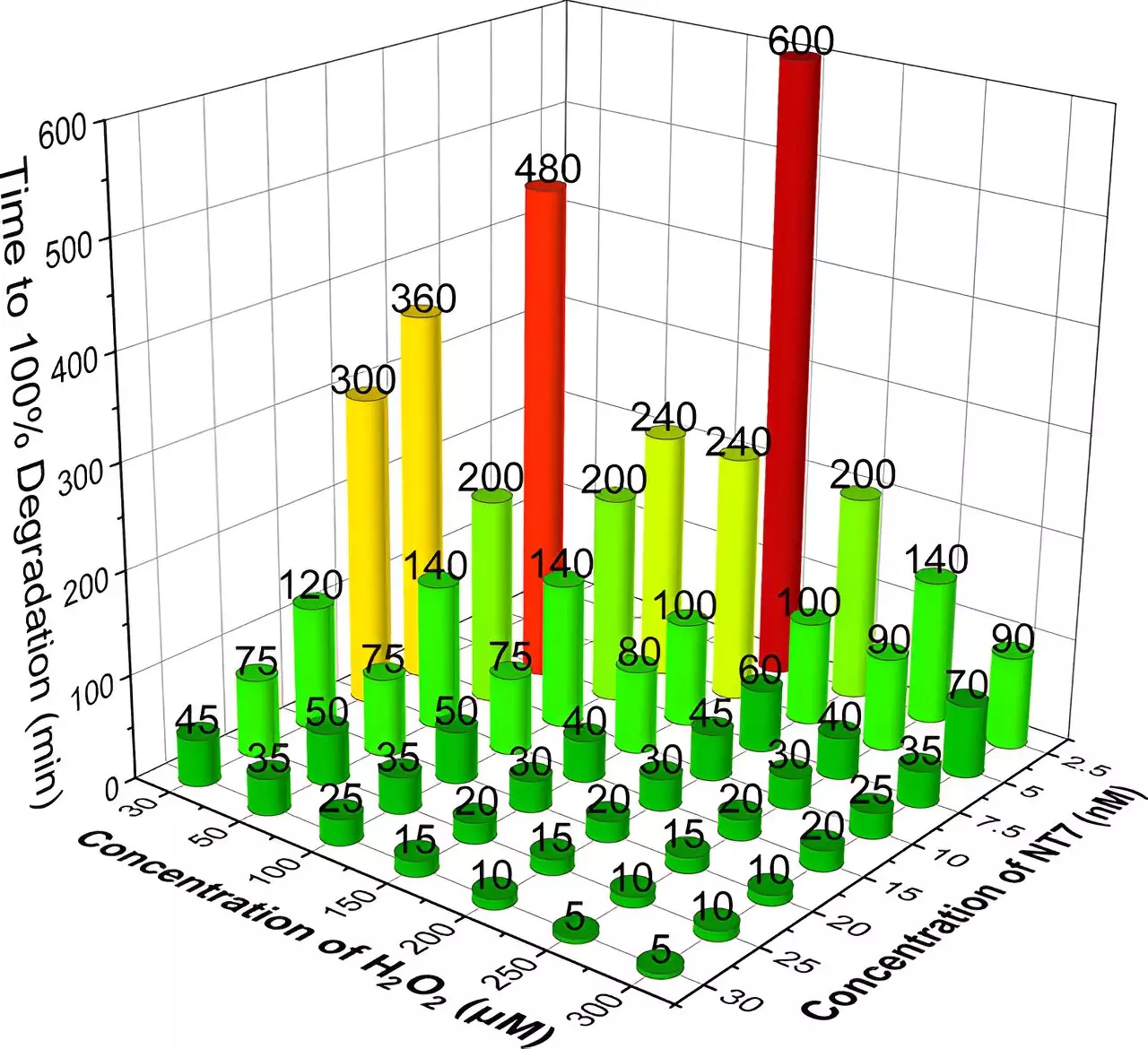
At the forefront of this research is the innovation of TAML (Tetra-Amido Macrocyclic Ligand) catalysts. Developed over decades by a team at Carnegie Mellon University, TAML catalysts are bioinspired miniatures of naturally occurring peroxidase enzymes. The latest iteration, known as NewTAML, demonstrates remarkable effectiveness in catalyzing hydrogen peroxide for the degradation of pharmaceuticals. The research led by Terry Collins, director of the Institute for Green Science, illustrates the simplicity of this process: mixing ultra-dilute solutions of TAML and hydrogen peroxide with contaminated water yields impressive results.
Collins emphasizes the cost-effectiveness and sustainability of this method, noting that the ultra-low concentrations of the catalyst and peroxide lead to significant reductions in operational costs. Additionally, the unique behavior of the TAML catalyst means that as its concentration decreases, its efficacy increases, allowing for substantial savings without compromising effectiveness.
In conducting experiments, the Carnegie Mellon team assessed the ability of NewTAML to break down six high-priority drugs, including common antibiotics and a synthetic estrogen. Their findings indicate that the process is not only effective in lab environments but also under real-world conditions, such as with municipal secondary wastewater and natural water sources. After thorough testing, results demonstrated that five of the six pharmaceuticals became undetectable, while the degradation of ciprofloxacin reached nearly complete removal at 95.4%.
This research paves the way for field applications, indicating the potential for a broader deployment of the TAML/peroxide system. The straightforward nature of the method—requiring little more than a mix of TAML and peroxide into contaminated waters—suggests that it can be widely adopted in various contexts, providing a viable solution for towns and cities still battling pharmaceutical contamination.
As the environmental burden of micropollutants continues to worsen, the urgency for effective wastewater treatment solutions cannot be overstated. Collins and his team have patented their advanced TAML formulations, which are now licensed to Sudoc, Inc., a startup poised to introduce these TAML-based solutions to the market. Sudoc has recently raised $20 million to facilitate the rollout of their technology, aiming to penetrate the European water treatment sector.
Next steps in the process include amplifying testing to real-world field applications, assessing not just efficacy but also the overall impact on both water quality and local ecosystems. The success of these efforts may not only lead to cleaner water resources but could also provide scalable solutions applicable in various regions facing similar challenges with pharmaceutical micropollutants.
Carnegie Mellon University’s innovative approach using TAML catalysts represents a significant stride toward combatting pharmaceutical pollution in wastewater. By prioritizing cost-effectiveness and environmental sustainability, this research offers a promising path for municipalities and industries to better manage micropollutants in water sources. As scientific and market efforts progress, the hope is that such solutions can be implemented globally, contributing to healthier ecosystems and safer drinking water for all.

Leave a Reply