Antimicrobial resistance (AMR) has become a pressing global public health crisis, as many modern drugs have become ineffective against drug-resistant bacteria. In response to this urgent challenge, a team of researchers from Harvard University has developed a groundbreaking antibiotic that overcomes various mechanisms of antimicrobial resistance. Led by Andrew Myers, a renowned professor of Chemistry and Chemical Biology, the team has successfully created a synthetic compound called cresomycin, which demonstrates the ability to kill drug-resistant strains of bacteria that cause severe infections. This scientific breakthrough could potentially save millions of lives worldwide.
Cresomycin has shown significant improvement in inhibitory activity compared to clinically approved antibiotics. These improvements are especially crucial considering the alarming death toll caused by bacterial infections each year. While further research is required to determine the safety and effectiveness of cresomycin in human subjects, the preliminary results offer a glimmer of hope in the ongoing battle against superbugs.
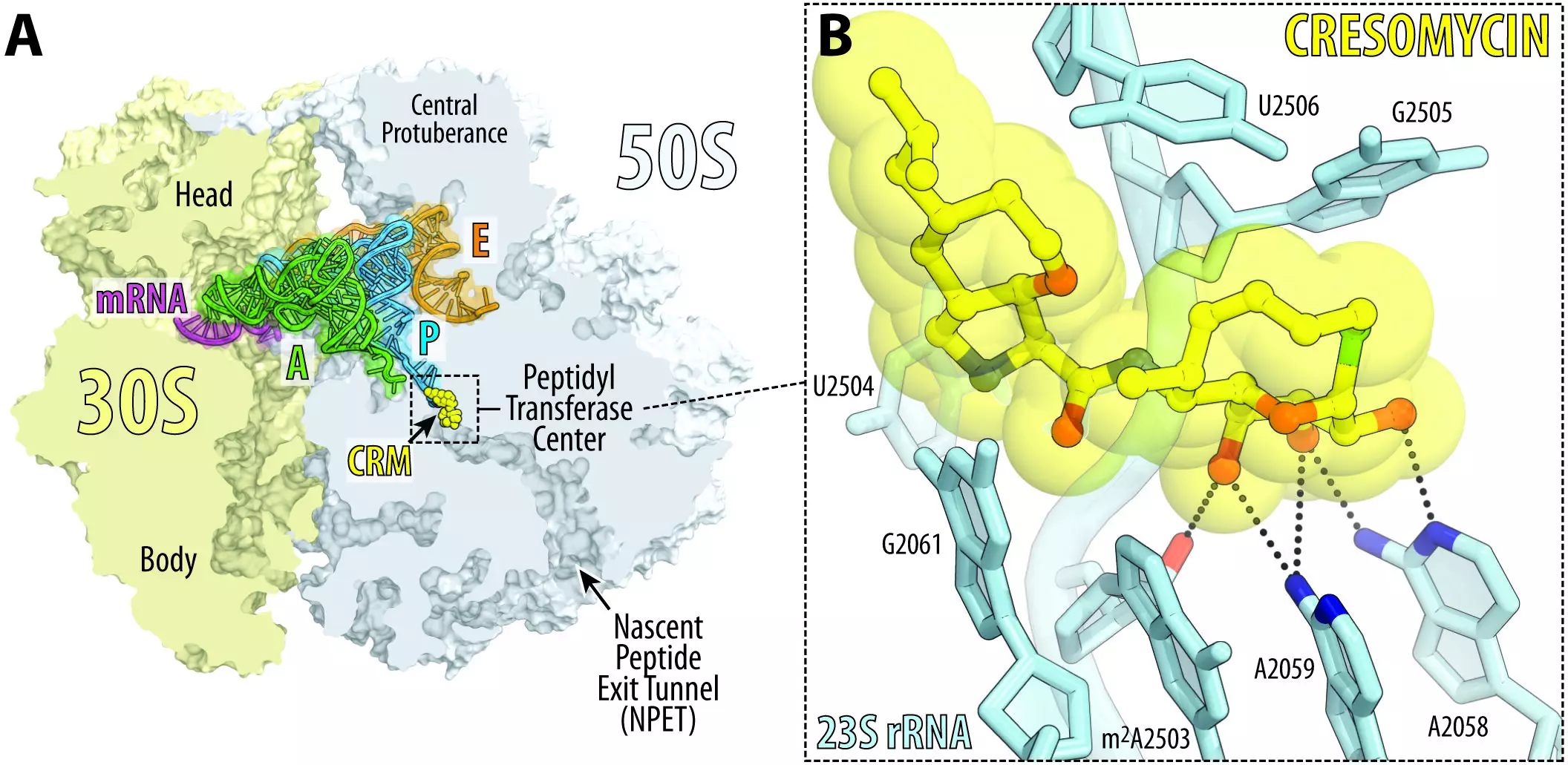
The Harvard team’s approach to antibiotic development is innovative and novel. Drawing inspiration from the chemical structures of lincosamides, a class of antibiotics that includes the widely prescribed clindamycin, cresomycin is fully synthetic and features chemical modifications not achievable through existing means. By leveraging the power of organic synthesis, the researchers have expanded the possibilities of antibiotic design, pushing the boundaries of what is currently feasible.
One of the primary reasons antibiotics become ineffective is the ability of bacteria to develop resistance mechanisms, such as producing enzymes called ribosomal RNA methyltransferases. These enzymes shield the drug components that disrupt bacterial ribosomes, rendering the drugs useless. To overcome this challenge, the Harvard researchers engineered cresomycin into a shape that closely resembles its binding target, allowing for a stronger grip on the ribosome. Termed as “pre-organized” for ribosomal binding, the drug does not need to expend as much energy conforming to its target, providing a distinct advantage over existing antibiotics.
The team’s success is attributed to their revolutionary approach to drug discovery. Through component-based synthesis, a method pioneered by the Myers lab, the researchers were able to build large molecular components of equal complexity and assemble them at later stages. This modular, fully synthetic system allowed for the creation and testing of a multitude of target molecules. This accelerated drug discovery process marks a significant step forward in combating antibiotic resistance and provides hope for a future with more effective treatments.
Read More: The Impact of Passing Stars on Earth’s Climate
Given the crucial role antibiotics play in modern medicine, the development of new and effective antibiotics is of utmost importance. Antibiotics form the foundation upon which modern medicine is built, and without effective drugs to combat bacterial infections, countless medical procedures and treatments could become extremely risky or even impossible. With the rise of drug-resistant bacteria and the limited availability of effective antibiotics, it is imperative that researchers continue to push the boundaries of antibiotic design and discovery.
The development of cresomycin by Harvard researchers offers a ray of hope in the fight against antimicrobial resistance. This synthetic compound not only kills drug-resistant bacteria but also demonstrates improved inhibitory activity compared to existing antibiotics. By redefining antibiotic design and revolutionizing the drug discovery process, the researchers have taken a significant step towards winning the war against superbugs. However, further research is necessary to evaluate the safety and efficacy of cresomycin in human subjects. Nevertheless, this groundbreaking medical breakthrough holds tremendous promise and potential to save lives and alleviate the global healthcare burden posed by antimicrobial resistance.

Leave a Reply