The search for sustainable and efficient methods to convert biomass into valuable chemicals has long fascinated researchers. Among several methodologies, the conversion of biomass into olefins—a critical precursor for plastics, pharmaceuticals, and other industrial materials—stands out for its economic and environmental implications. Recent research by a team from Kyushu University has shed light on a promising breakthrough using Na-ZSM-5, a zeolite material, in conjunction with microwave heating, marking a significant step toward revolutionizing the chemical industry.
Historically, the reforming of naphtha has served as a primary method for synthesizing necessary precursor chemicals. However, this traditional approach is fraught with limitations; it not only demands high energy inputs but also contributes significantly to carbon dioxide emissions. Efforts have thus turned to alternative resources such as wasted cooking oils and microalgal oils. These renewable materials can be converted into simpler chemicals through catalytic cracking, a process that can also be powered by zeolite catalysts.
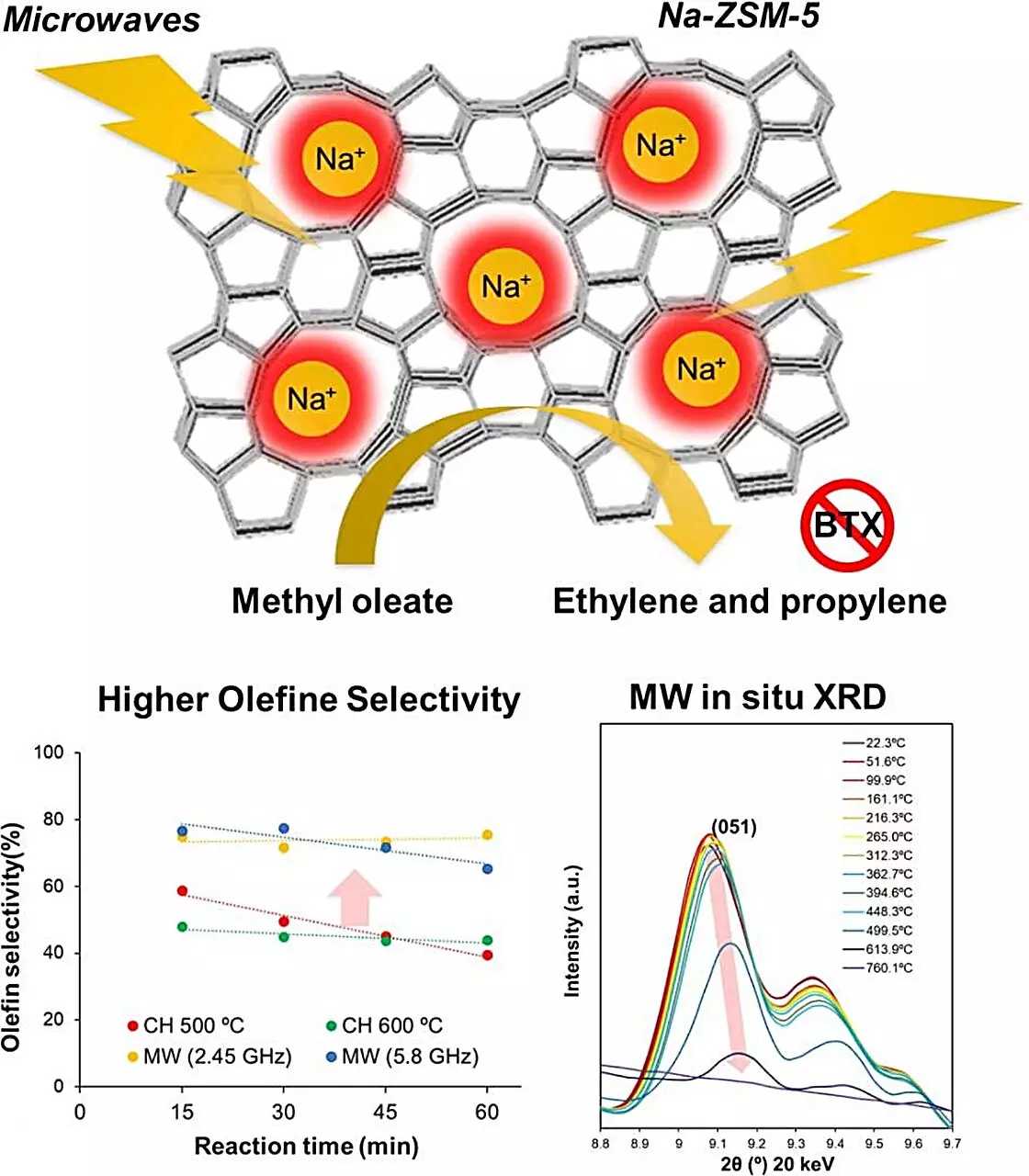
Zeolites, characterized by their porous structure, are integral to the catalytic cracking process. Generally, temperatures of 500–600°C are required for effective conversion. Unfortunately, operating at these high temperatures poses a dual challenge: significant energy consumption and coking, where unwanted deposits diminish catalyst effectiveness over time. This study led by Associate Professor Shuntaro Tsubaki highlights microwave-assisted heating as a feasible alternative to traditional methods, diminishing the adverse effects seen with high-temperature thermal processes.
Microwave energy serves as a unique tool in this context, allowing for direct interaction with materials and enabling targeted heating, which can lead to considerable energy savings. Tsubaki explains, “Microwaves can accelerate gas–solid catalysis by traversing the gas phase and heating the solid catalyst selectively,” thus overcoming some of the inherent challenges faced with conventional heating methods.
In their investigation, Tsubaki and his team meticulously examined various zeolite materials to pinpoint which ones would perform best under microwave heating conditions. Through both theoretical models and practical experimentation, they identified Na-ZSM-5 as the most effective candidate. This sodium ion-substituted zeolite demonstrated remarkable catalytic performance, particularly in the conversion of fatty acid esters into olefins when subjected to microwave radiation.
Notably, the use of microwave heating resulted in a staggering increase in olefin production—four times higher than traditional heating methods at identical temperatures. Additionally, the production of greenhouse gases like carbon dioxide was dramatically minimized, showcasing microwave heating’s potential for significant environmental benefits.
To further understand the superiority of microwave heating, the researchers examined the structural changes of Na-ZSM-5 under microwave irradiation. Surprisingly, they discovered that localized temperatures within the zeolite’s crystalline structure could surge to over 1000°C, while the bulk material remained at a stable 500°C. This phenomenon likely facilitates the selective formation of olefins, marking a crucial finding that could accelerate advancements in catalytic processes.
The implications of these findings are profound. By adopting such innovative techniques, the chemical industry can enhance biomass conversion efficiency and work toward environmental sustainability. Tsubaki emphasizes, “Our findings are expected to contribute to the electrification of the chemical industry,” as microwave technology can harness renewable energy sources, thereby reducing production-related carbon footprints.
Looking ahead, the researchers plan to refine their microwave-driven catalytic processes to further optimize yield and energy efficiency. Scalability remains a critical aspect, and ongoing efforts aim to streamline these advancements to foster widespread adoption across the chemical manufacturing sector.
The Kyushu University study illustrates how microwave-enhanced zeolite catalysts like Na-ZSM-5 could pave the way for more sustainable chemical processes. As the pressure to minimize environmental impacts grows, innovations in energy-efficient biomass conversion will be key to meeting the evolving demands of both industry and the planet.

Leave a Reply