Breaking new grounds in the field of chemical research, scientists from Tokyo Tech have discovered a groundbreaking method that involves immobilizing small synthetic molecules inside protein crystals to study intermediate compounds formed during chemical reactions. By combining this innovative approach with time-resolved serial femtosecond crystallography, they have successfully visualized reaction dynamics and rapid structural changes occurring within reaction centers immobilized inside protein crystals.
The Importance of Studying Intermediate Compounds
Most complex chemical reactions, whether synthetic or biological, do not involve a direct transformation of reactants into products. Instead, they often proceed through the formation of short-lived intermediate compounds that undergo further reactions until the final products are obtained. Understanding these stepwise processes in detail is crucial for advancements in fields such as energy generation, catalysis, and medicine.
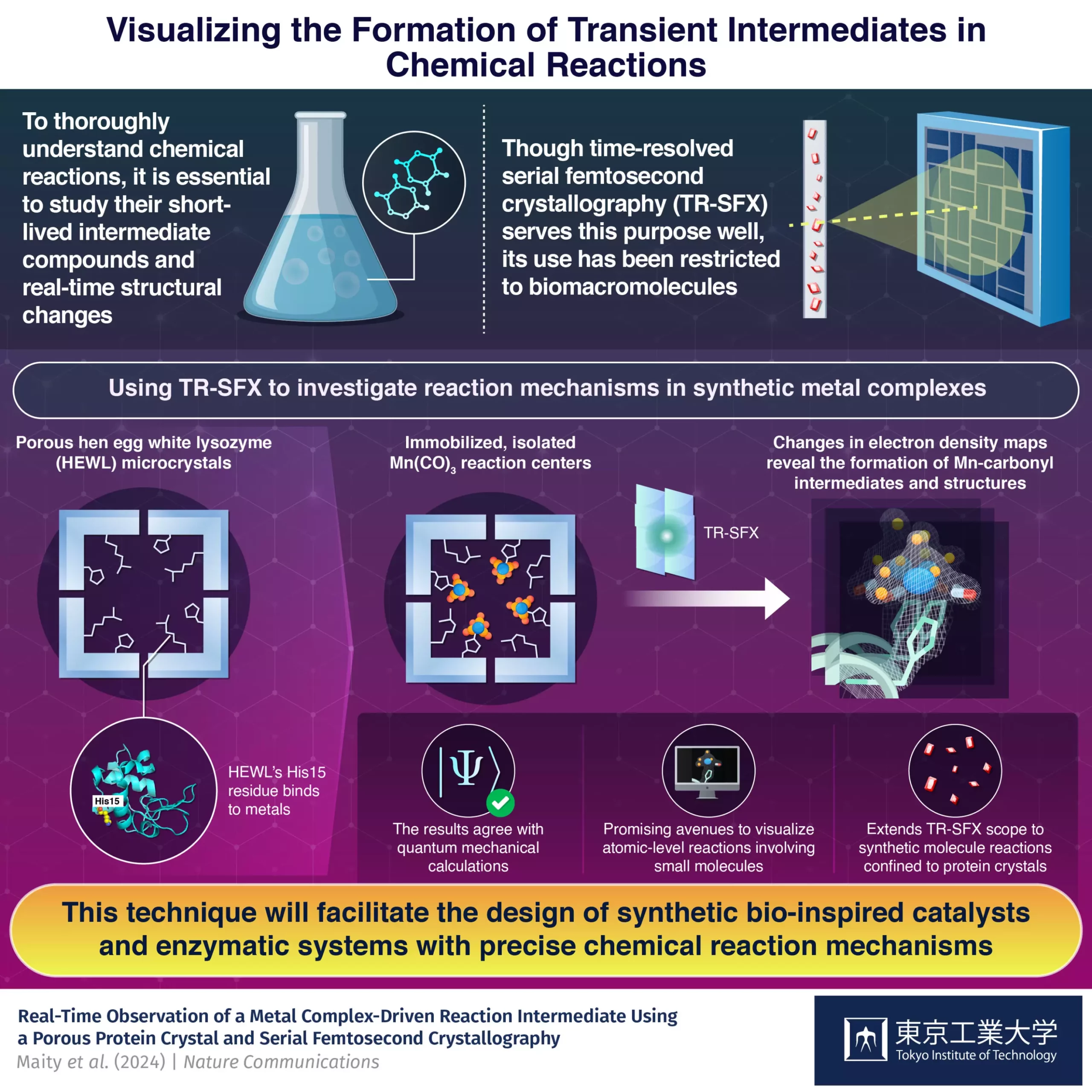
Visualizing short-lived intermediates in chemical reactions is quite challenging, especially when one needs to capture structural changes in a molecule at the atomic level. One advanced method to achieve this is time-resolved serial femtosecond crystallography (TR-SFX), which involves shooting extremely fast electron laser pulses at crystallized molecular structures and capturing the diffraction patterns to record reaction dynamics.
Expanding the Applications of TR-SFX
Although TR-SFX has primarily been limited to biomacromolecules, a research team led by Professor Takafumi Ueno from Japan decided to showcase the technique’s potential for analyzing reactions in synthetic compounds. They successfully captured the dynamics of carbon monoxide (CO) release from Mn(CO)3, highlighting the versatility of TR-SFX in studying non-biological molecules.
One significant limitation that the researchers had to overcome is the preference of TR-SFX for microcrystals, typically found in biomacromolecules. To address this issue, the team developed a strategy using hen egg-white lysozyme (HEWL), a protein that naturally crystallizes into a nanoporous structure suitable for TR-SFX. By immobilizing a light-sensitive Mn(CO)3-containing compound inside HEWL crystals, they created an ideal environment for studying CO release reactions.
Experimental Findings and Future Implications
The experimental results obtained were in excellent agreement with quantum mechanical calculations, validating the proposed strategy. This method paves the way for the intelligent design of drugs, catalysts, and enzymatic systems involving non-biological components. It opens up new possibilities for studying synthetic metal complexes and designing artificial metalloenzymes with precise mechanisms, enabling the development of innovative reactions in the future.
The integration of small molecules into protein crystals represents a significant advancement in chemical research. By leveraging the capabilities of TR-SFX and combining them with innovative strategies, researchers are now able to visualize and analyze complex reaction dynamics at the atomic level. This approach not only furthers our understanding of chemical processes but also provides a platform for the design and development of novel drugs, catalysts, and functional materials.

Leave a Reply