A recent collaboration led by Kyriakos Stylianou at Oregon State University has resulted in a significant breakthrough in the conversion of sunlight and water into clean energy. The researchers have successfully developed a new material that acts as a potent photocatalyst, enhancing the rapid and efficient production of hydrogen. This hydrogen can be utilized in various applications, including fuel cells for cars, chemical manufacturing, metal refining, and plastics production.
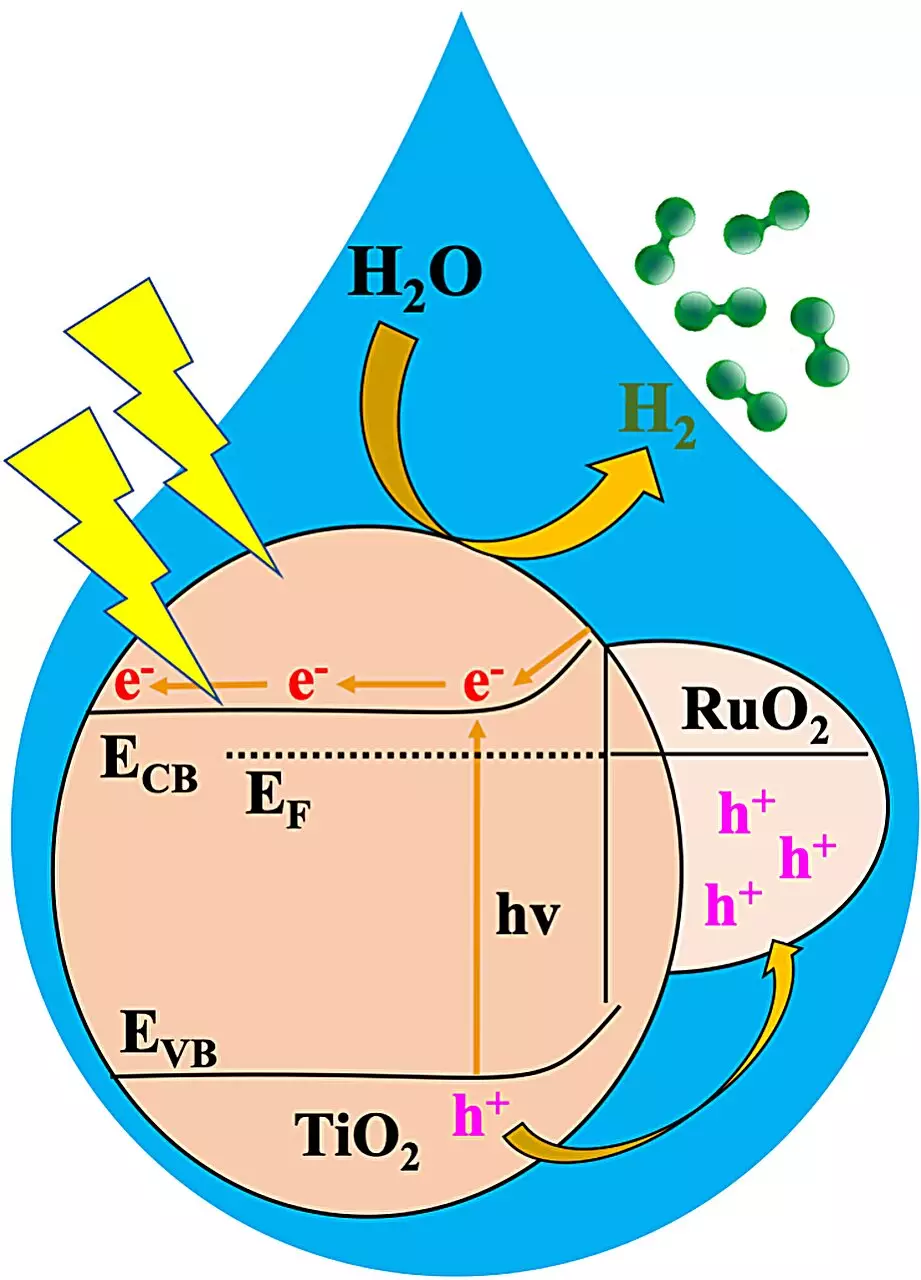
The key to this groundbreaking material lies in metal organic frameworks, also known as MOFs. These crystalline, porous materials are composed of positively charged metal ions surrounded by organic linker molecules. MOFs possess nanosized pores and adjustable structural properties, making them highly versatile in design and application. In this specific study, researchers utilized a MOF to create a metal oxide heterojunction, combining ruthenium oxide and titanium oxide doped with sulfur and nitrogen.
The resulting heterojunction, referred to as RTTA, demonstrated exceptional performance in splitting water into hydrogen when exposed to sunlight. Among the various RTTA materials tested, RTTA-1 stood out with the lowest ruthenium oxide content, showcasing the highest hydrogen production rate and quantum yield. In just one hour, a gram of RTTA-1 was able to produce over 10,700 micromoles of hydrogen, utilizing photons at an impressive rate of 10%. This remarkable activity is attributed to the synergistic effects of the metal oxides’ properties and the surface properties derived from the parent MOF, enhancing electron transfer.
The process of producing hydrogen through the catalytic splitting of water offers significant advantages over conventional methods, such as methane-steam reforming. Electrolysis, the current method for hydrogen production from water, requires electricity to run through the catalyst. This approach relies on renewable energy sources for sustainability, emphasizing the importance of cost-effectiveness in the market. Green hydrogen produced through water splitting currently costs approximately $5 per kilogram, compared to $1.50 per kilogram for hydrogen derived from methane-steam reforming.
The development of MOF-derived metal oxide heterojunctions as photocatalysts presents a promising pathway towards practical and efficient hydrogen production. By leveraging the abundance of water as a source of hydrogen and harnessing solar energy through photocatalysis, the research team led by Stylianou is contributing to the advancement of sustainable and clean energy solutions. While the use of ruthenium oxide in the photocatalyst may raise concerns regarding cost, the minimal amount required underscores the feasibility and practicality of this innovative approach.
The recent advancements in clean energy production through innovative research reflect a significant step towards a sustainable future. The utilization of MOFs and metal oxide heterojunctions as photocatalysts showcases the potential for revolutionizing hydrogen production with high efficiency and minimal environmental impact. As renewable energy sources continue to gain traction in the market, the development of practical solutions for clean energy generation is paramount in addressing climate change and reducing greenhouse gas emissions.

Leave a Reply