Samarium (Sm), although considered a rare earth metal, plays a crucial role in the world of organic synthesis. Its ability to engage in single-electron transfer reactions, particularly through its divalent compounds, opens up a broad spectrum of possibilities for organic chemists, enabling the reduction of various organic compounds. Among the numerous Sm compounds, SmI2 stands out due to its moderate stability and effectiveness under benign conditions—attributes that significantly contribute to its utility in the pharmaceutical landscape. The versatility of SmI2 makes it a prime candidate for diverse synthetic applications, paving the way for the development of biologically active materials crucial to modern medicine.
Despite the advantages of using Samarium, the conventional methods of employing SmI2 often come with considerable drawbacks. Chief among these issues is the requirement for high stoichiometric amounts of Sm, which leads to increased costs and a resource-intensive process. Notably, conventional reactions frequently necessitate the use of toxic chemicals that pose additional safety and environmental concerns. The pursuit of reducing the Sm agent to catalytic levels has been an area of active research, yet many existing methodologies still rely on harsh conditions and aggressive reducing agents, resulting in the utilization of 10–20% of Sm relative to the starting materials. This excessive demand is a significant barrier to the widespread application of Sm-based reactions in an economically and environmentally sustainable manner.
A Novel Approach to Catalysis
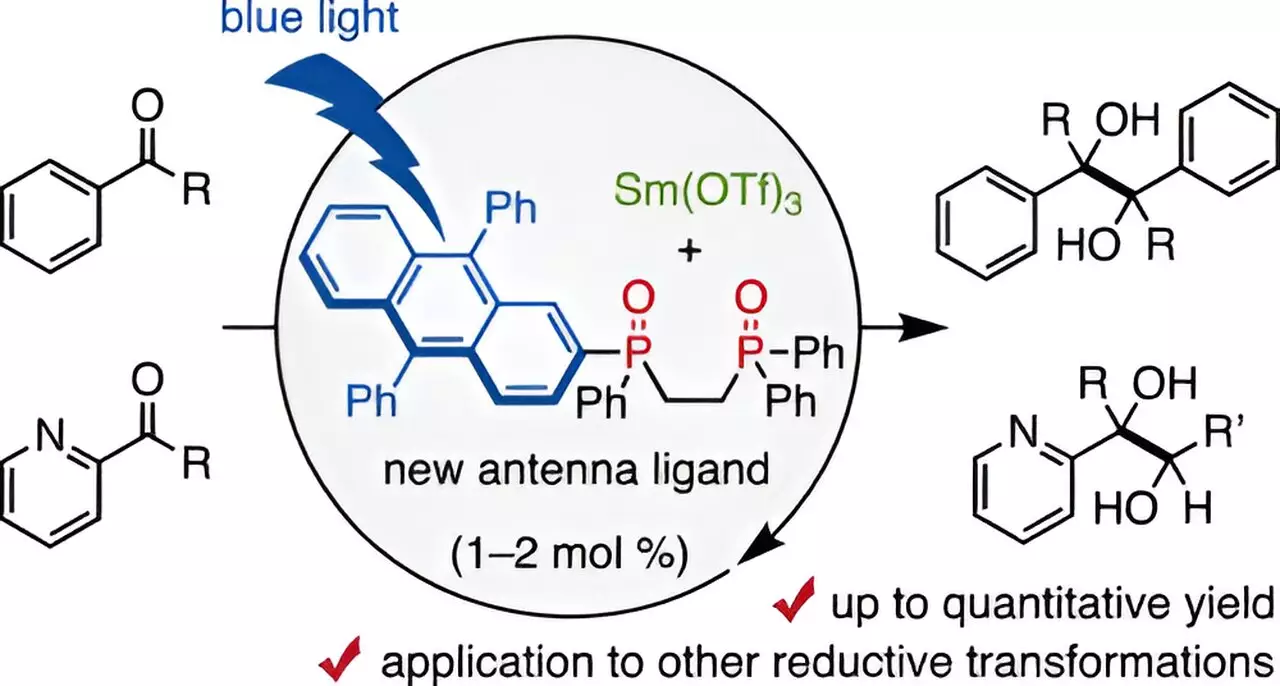
In a noteworthy advancement, a team of researchers at Chiba University, led by Assistant Professor Takahito Kuribara, introduced a transformative approach that dramatically decreases the reliance on Sm in organic synthesis. Their innovative study, published in the Journal of the American Chemical Society, showcases the development of a bidentate phosphine oxide ligand, specifically a 9,10-diphenyl anthracene (DPA)-substituted variant. This unique ligand facilitates coordination with trivalent samarium, creating a system where visible light can be harnessed to spur Sm-catalyzed reductive processes. This adaptation marks a significant departure from the conventional protocols that often involve cumbersome setups and harsh reagents.
The Role of the Visible-Light Antenna
Assistant Professor Kuribara emphasizes the potential of the newly designed ligand, which can act as a visible-light antenna, exciting the inherently stable lanthanoid metal such as Sm. Drawing inspiration from previous research involving a secondary phosphine oxide ligand that demonstrated effective reduction-oxidation capabilities under visible light, the team succeeded in fine-tuning a more efficient bidentate variant. The resulting DPA-1 ligand not only lowers the amount of Sm required for catalytic action to as little as 1–2 mol% but also improves the efficiency of catalytic reactions significantly.
Experimental Successes and Applications
The practical implications of this research are striking. By combining Sm with DPA-1 and exposing it to blue light, the team reported impressive yields of up to 98% in pinacol coupling reactions of aldehydes and ketones—substrates frequently encountered in pharmaceutical synthesis. This innovative approach circumvents the need for conventional high-loading strategies and opens the door for reactions to proceed with much milder organic reducing agents. Furthermore, exploratory experiments indicated that the optimal solvent conditions included small amounts of water, enhancing yields, while excess water proved counterproductive.
The versatility of the DPA-1 and Sm combination has also been evaluated across various molecular transformation reactions. Success in carbon-carbon bond formations and both carbon-carbon and carbon-oxygen bond cleavages not only underscores the practical applications in drug development but also demonstrates the ligand’s role in facilitating complex organic reactions.
The advances described in this research illustrate a shift in how chemists can leverage rare earth metals within organic reactions. By utilizing visible light as an energy source, the study enriches the existing toolkit available for synthetic chemists. This new method not only maintains the integrity of trivalent Sm, which is more manageable than its divalent counterpart, but also reduces operational costs associated with these types of reactions.
The research conducted by Kuribara and his colleagues offers exciting new pathways to enhance the efficiency and sustainability of Sm-catalyzed transformations. This shift toward minimal Sm inputs under benign conditions not only has the potential to revolutionize the field of organic chemistry but could also lead to more eco-friendly practices in drug synthesis, an important consideration for the future of pharmaceutical development.

Leave a Reply