Pancreatic cancer is notorious for being one of the deadliest forms of cancer due to its late diagnosis. The current markers used for screenings are not sensitive or specific enough for early detection. This is a major problem in the field of cancer research, as early detection is crucial for successful treatment and improved patient outcomes.
In a recent study published in the journal Angewandte Chemie, a research team has introduced a groundbreaking method for the more precise and reliable diagnosis of pancreatic cancer. This method is based on the selective detection of specific antibodies in blood samples. Tumors produce certain proteins known as tumor-associated antigens that trigger an immune response and lead to the formation of tumor-associated autoantibodies in the bloodstream.
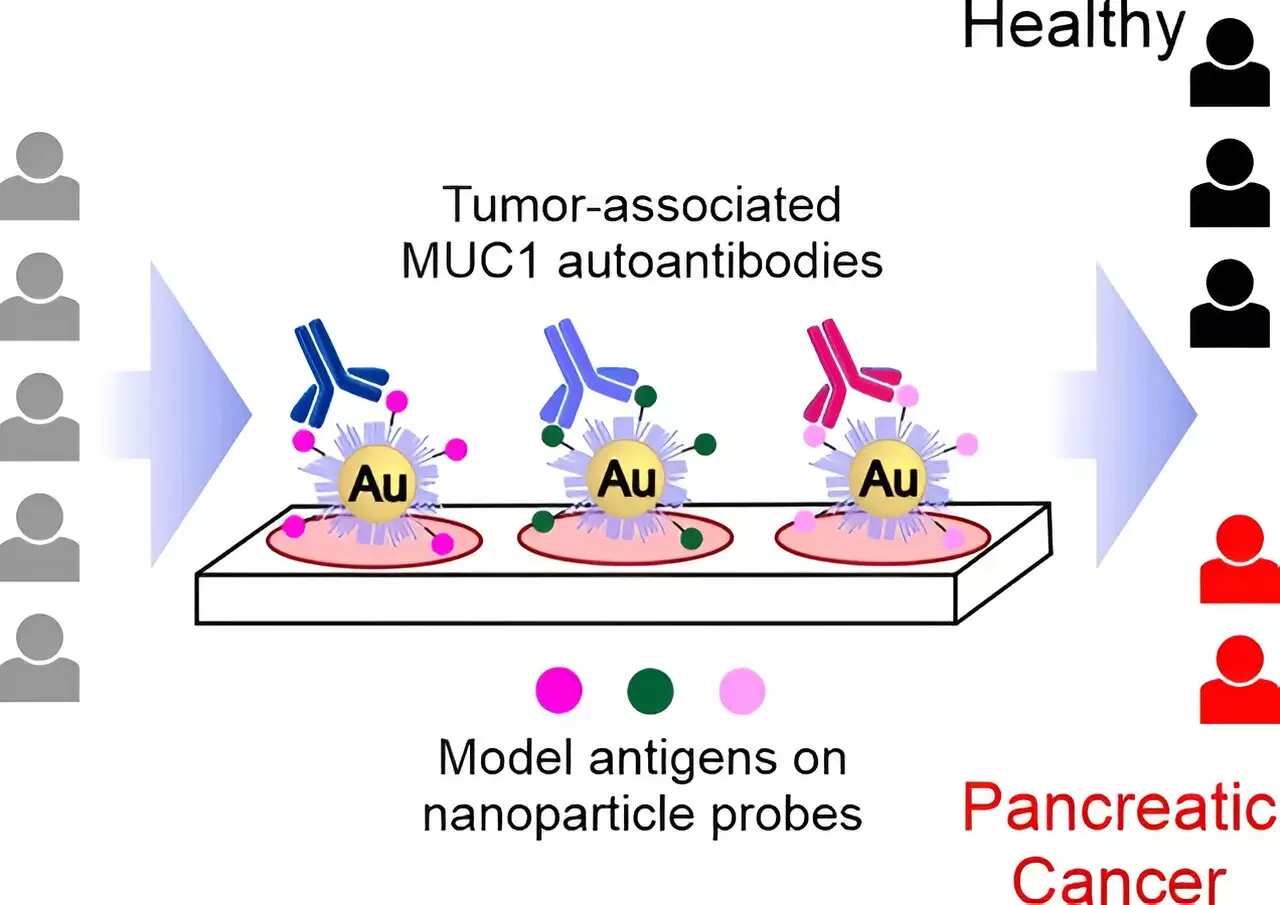
Led by researchers at the University of Verona in Italy and the Center for Biomedical Research of La Rioja in Spain, the team focused on detecting autoantibodies directed against the tumor-associated form of mucin-1 (TA-MUC1). Mucin-1 is a protein commonly found in glandular tissue, with significantly elevated concentrations in various types of tumors, including pancreatic cancer. The team’s goal was to identify autoantibodies that specifically target TA-MUC1 as a clear indicator of pancreatic cancer.
Through structural analyses and computer simulations, the researchers designed a collection of synthetic glycopeptides that mimic different segments of TA-MUC1. These glycopeptides were then immobilized on gold nanoparticles to create probes suitable for a serological assay. The diagnostic assay was tested using samples from patients with pancreatic cancer and a healthy control group. The nanoparticle probes were able to differentiate effectively between samples from diseased and healthy individuals, demonstrating the detection of tumor-associated autoantibodies.
The study found that probes with smaller glycopeptide antigens corresponding to a single epitope performed better than larger probes mimicking multiple epitopes. This not only improved the accuracy of the test but also simplified the synthetic production of the probes. A short glycopeptide with an unnatural modification to its sugar component was particularly effective in detecting discriminating autoantibodies. This structured-based approach has the potential to select autoantibody subgroups with higher tumor specificity, improving the early detection of pancreatic cancer.
Overall, this innovative method of selective antibody detection offers promising prospects for revolutionizing the diagnosis of pancreatic cancer. By focusing on tumor-associated autoantibodies and utilizing synthetic glycopeptides as probes, researchers have paved the way for more precise and reliable early detection screenings. This breakthrough could potentially save countless lives by enabling earlier interventions and personalized treatment strategies for patients with pancreatic cancer.

Leave a Reply