The researchers at the University of Liverpool have made significant progress in converting carbon dioxide (CO2) into valuable fuels and chemicals. In their recent work published in the journal Chem, the team introduced a novel plasma-catalytic process for the hydrogenation of CO2 to methanol at room temperature and atmospheric pressure. This groundbreaking approach overcomes the limitations of traditional thermal catalysis, which typically requires high temperatures and pressures, resulting in low CO2 conversion and methanol yield.
The innovative process incorporates a bimetallic Ni-Co catalyst within a non-thermal plasma reactor. This unique setup enables the researchers to achieve an impressive single-pass 46% selectivity for methanol and 24% CO2 conversion at only 35 degrees Celsius and 0.1 MPa. Non-thermal plasma, characterized by an ionized gas containing energetic electrons and reactive species, plays a crucial role in activating strong chemical bonds of inert molecules like CO2. This activation facilitates chemical reactions under mild conditions, paving the way for greater efficiency in CO2 conversion processes.
Professor Xin Tu, Chair in Plasma Catalysis at the University of Liverpool, emphasizes the flexibility and decentralized nature of plasma catalysis in CO2 hydrogenation. The team’s recent techno-economic assessment demonstrates that this new process significantly reduces capital costs compared to traditional thermal catalytic methods. By utilizing renewable energy sources in the production of synthetic fuels, plasma catalysis emerges as a viable solution for a sustainable future.
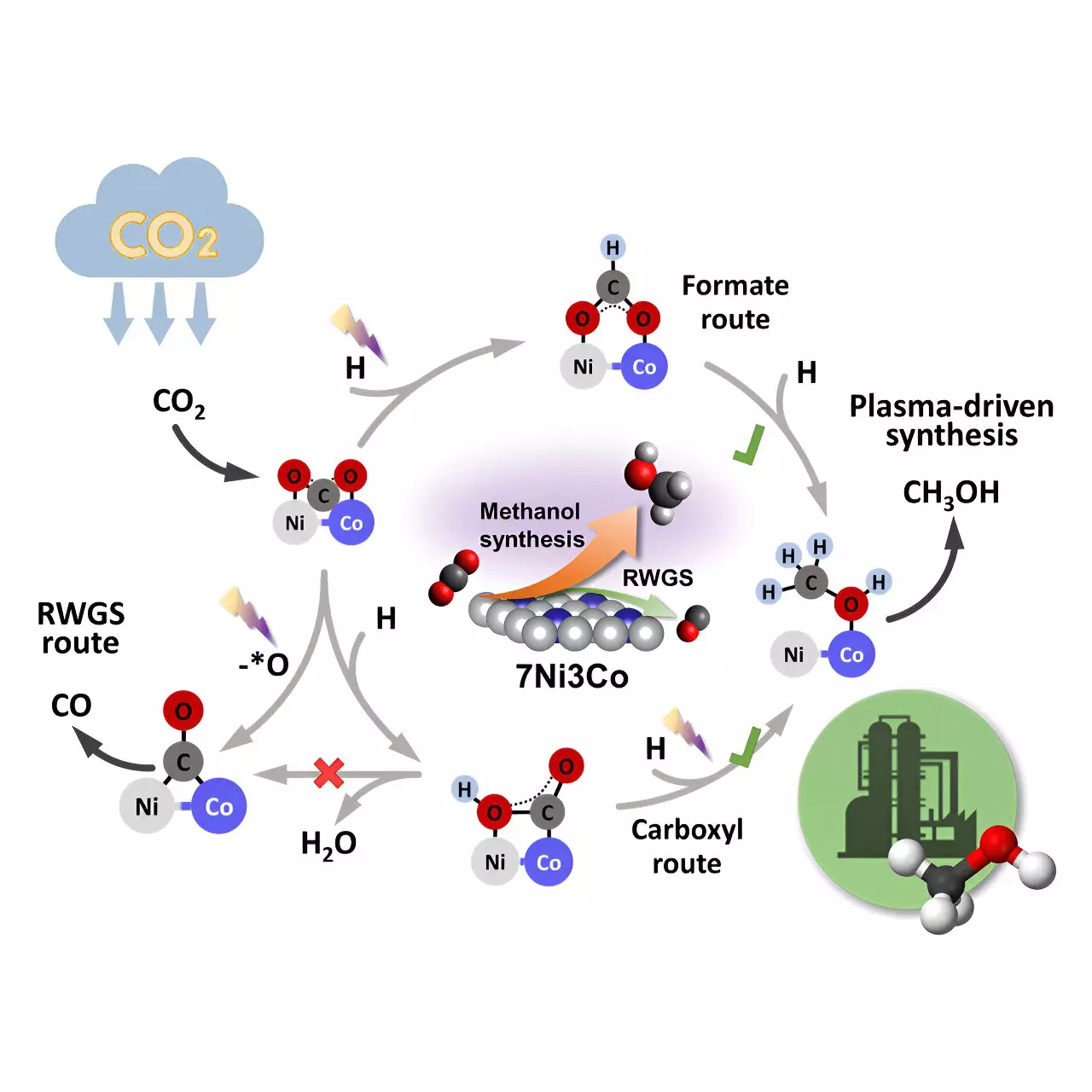
In-depth characterization techniques such as in situ plasma-coupled Fourier transform infrared (FTIR) and density functional theory (DFT) calculations shed light on the mechanism behind methanol synthesis. The researchers found that the bimetallic Ni-Co interface serves as the primary active center for methanol production. CO2 adsorption and hydrogenation occur via the Eley-Rideal mechanism, generating various intermediates crucial for methanol formation. The formate and carboxyl routes were identified as significant pathways, while the reverse water-gas shift (RWGS) and CO hydrogenation were less favorable on the Ni-Co sites.
The precise control of Ni-Co sites in bimetallic catalysts holds immense promise for tailoring reaction pathways and product distribution. Plasma catalysis emerges as a key electrification technology for sustainable CO2 conversion and fuel production. The ability to conduct these reactions at ambient conditions using a scalable plasma system offers a compelling alternative for the chemical industry. Furthermore, powering plasma-based systems with intermittent renewable electricity enhances the feasibility of decentralized fuel and chemical production, paving the way for a more sustainable future.
The University of Liverpool research team’s pioneering work marks a major advancement in catalytic CO2 conversion. Their efforts present promising avenues for future research and industrial applications to address the global challenge of sustainability. As leaders in plasma catalysis, the research team has also made significant progress in other areas, such as CO2 methanation and biogas conversion to methanol. With three PCT patents filed in this area, the team continues to drive innovation in sustainable fuel and chemical production.
The University of Liverpool’s groundbreaking research in plasma catalysis for CO2 conversion signifies a significant step towards achieving a sustainable net-zero economy. By leveraging the unique capabilities of non-thermal plasma and bimetallic catalysts, the research team has unlocked new possibilities for efficient and environmentally friendly fuel and chemical production. Through continued innovation and collaboration, plasma catalysis holds the potential to revolutionize the way we approach CO2 conversion and contribute to a more sustainable future.

Leave a Reply