In a groundbreaking study conducted at the University of Illinois Urbana-Champaign, researchers have made significant strides in uncovering the role of solvation in ion binding and have introduced a novel pathway for electrochemically controlling ion selectivity. Published in JACS Au, this research builds upon previous work by Professor Xiao Su and Ph.D. student Raylin Chen, who have been exploring electrochemical separations of ions and have identified solvation as a critical mechanism for ion binding. By manipulating the solvation of a polymer through an electrochemical process, the researchers have successfully achieved specific ion binding with promising potential for future applications.
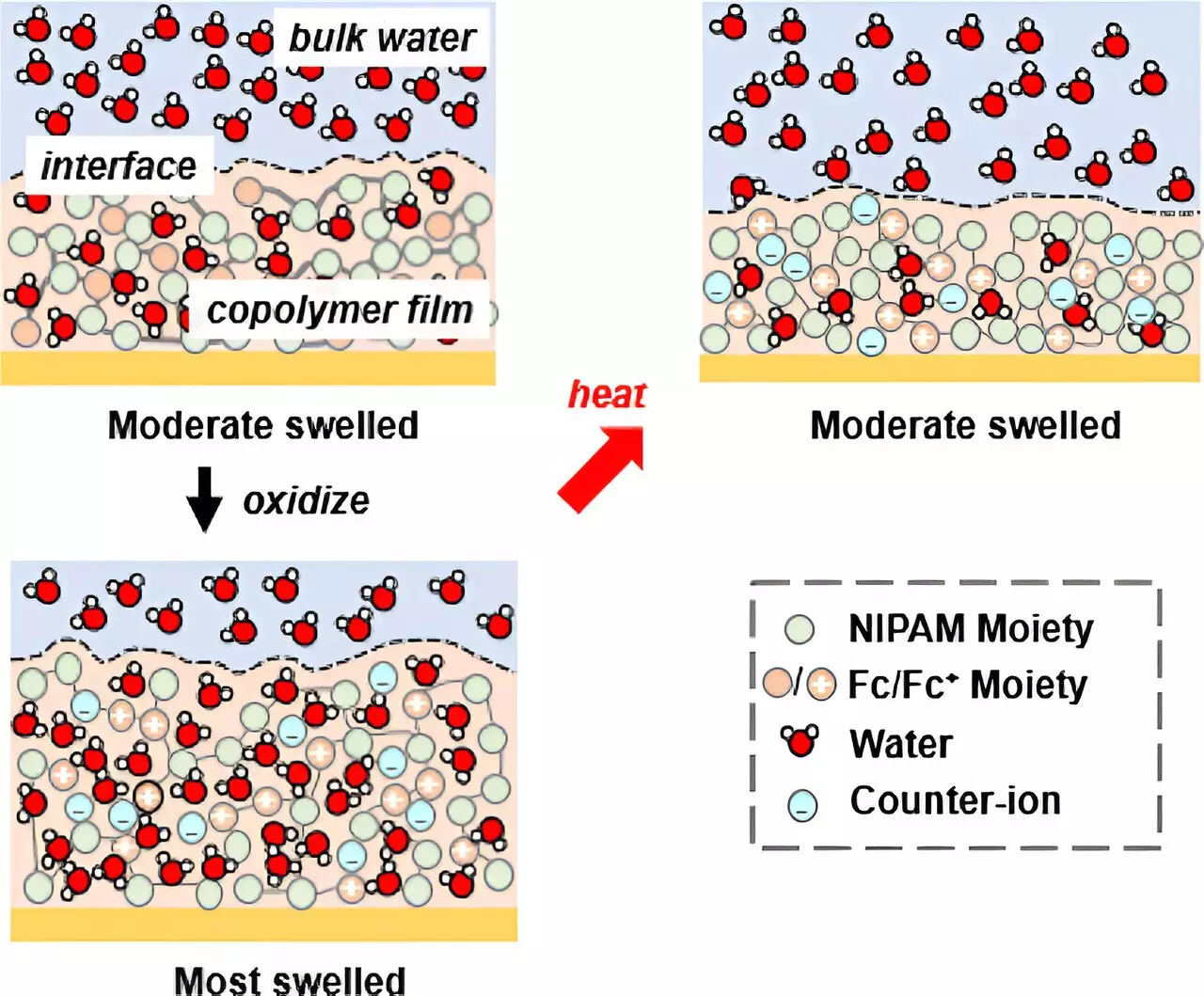
To achieve their objective, the research team developed a copolymer system that includes N-isopropyl acrylamide (NIPAM), known for its temperature-responsive properties, and introduced redox-active units. This unique combination provided two pathways to control solvation: the electroactive unit and the original thermo-responsive unit. By adjusting the potential, the researchers were able to influence the copolymer’s behavior, causing it to either retain or release water based on the electrochemical process. This electrochemical transition on NIPAM, rather than a traditional thermal transition, allowed for precise control over solvation.
Using the copolymer, the researchers fabricated gel films, which served as a platform for solvation-controlled ion separations. Through their developed in situ ellipsometry technique, they were able to observe the thickness of the film as it swelled or deswelled in response to the addition or release of water driven by electrochemistry. To gain a deeper understanding of solvation and water distribution within the film, the research team collaborated with Oak Ridge National Laboratory and utilized neutron reflectometry (NR), a technique sensitive to water content. NR provided valuable insights into the solvation process by revealing the amount of water distributed across the film. Under the influence of potential, the film demonstrated swelling behavior, indicating water uptake.
The researchers successfully demonstrated that the degree of water uptake by the film directly correlated to the control of ion selectivity. By varying the amount of water absorbed by the film, they were able to selectively bind different ions. This groundbreaking finding opens up possibilities for the development of more energy-efficient and selective technologies in water treatment and resource recovery. Moreover, the ability to activate the copolymer system through both temperature and electrochemical potential lays the foundation for future applications in various sustainable scenarios, including those powered by renewable energy or waste heat.
The innovative study conducted by the University of Illinois researchers significantly contributes to the field of electrochemical separations for water treatment and resource recovery. By providing a comprehensive understanding of the molecular mechanisms behind ion binding, this research paves the way for the development of materials platforms that offer precise control over ion binding mechanisms. The potential applications of this discovery are far-reaching and hold great promise for the future, particularly in the realms of sustainable energy and environmental remediation. As scientists continue to deepen their understanding of solvation and electrochemical control, we can anticipate further advancements in the field and the emergence of novel technologies that will lead to a more sustainable and efficient future.

Leave a Reply