Peptides are short chains of amino acids that have a wide range of applications in the fields of therapeutics, biomaterials, and chemical and biological research. One of the key challenges in working with peptides is the ability to attach functional molecules to them in specific locations without affecting their overall structure and function. Researchers have long been seeking methods to selectively modify peptides, particularly at the N-terminus, in order to enhance their utility in various applications.
In the past, attaching functional molecules to peptides, especially at the N-terminus, has been hindered by a number of limitations. These limitations include the release of functional groups in physiological conditions, the inability to attach multiple functional groups simultaneously, uneven attachment of functional molecules, and inefficient reaction processes. These challenges have made it difficult for researchers to modify peptides in a precise and controlled manner.
The Development of a New Chemical Reaction
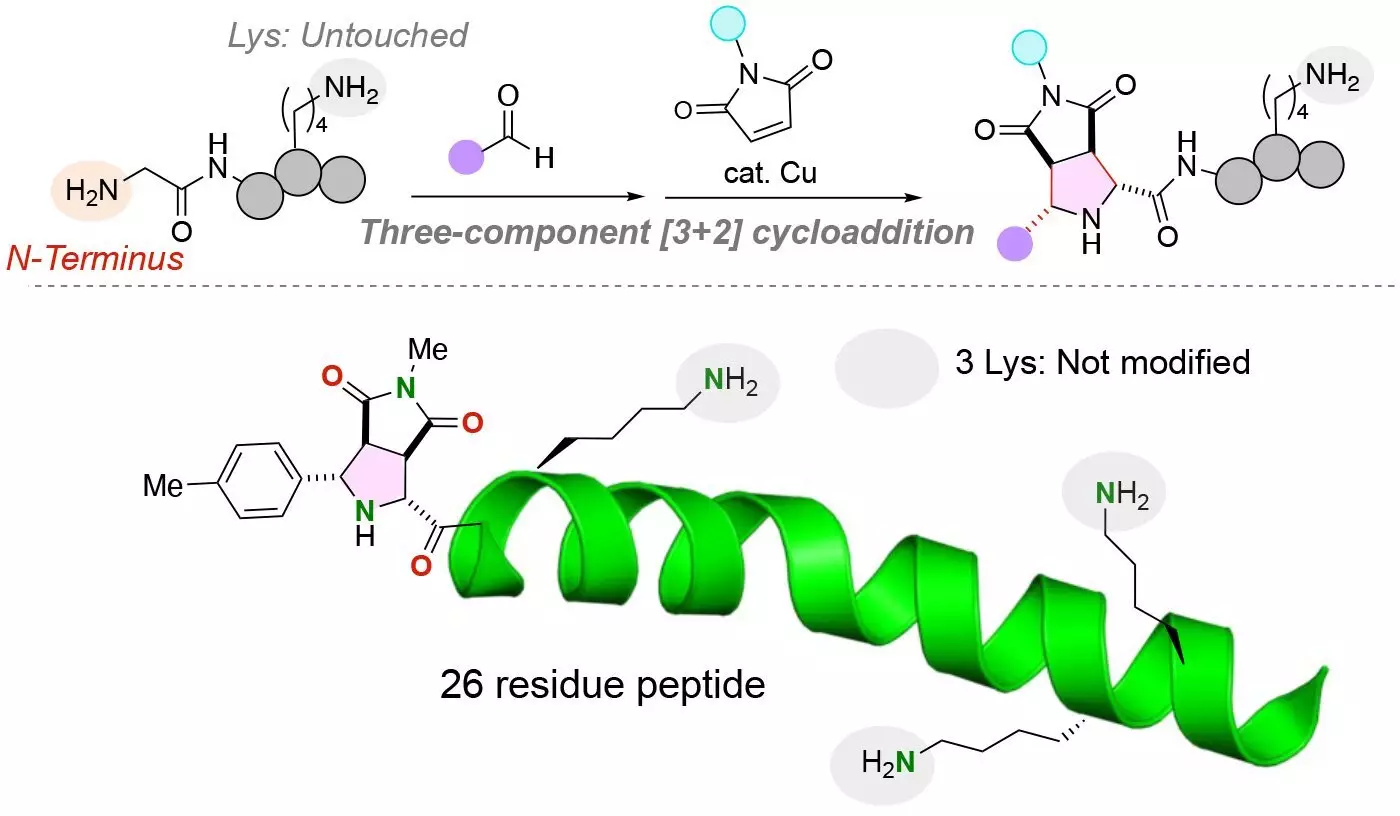
To address these limitations, researchers from Tohoku University and Chuo University have developed a novel chemical reaction for attaching two distinct functional molecules to the N-terminus of a peptide with a glycine amino acid at the N-terminus. This groundbreaking research, published in the journal Angewandte Chemie International Edition, demonstrates the ability to selectively modify peptides, regardless of the presence of reactive lysine residues, resulting in structurally uniform conjugates with high yields.
The key innovation of this research lies in the three-component protocol used by the researchers, which enables the simultaneous installation of two functional molecules into a peptide. By employing a copper catalyst in the reaction of peptides, aldehydes, and maleimides, the team was able to achieve stable carbon-carbon bonds between the N-terminus of the peptide and the functional molecules. This single-pot reaction takes place under mild conditions, making it highly efficient and effective.
Overcoming the Challenges Posed by Lysine Residues
One of the major challenges in modifying peptides is the presence of lysine residues, which have complicated the addition of functional molecules to the N-terminus. Lysine residues contain an amine group that can potentially compete with the amine group at the N-terminus of a peptide. However, the chemical reaction developed by the research team specifically labels only the N-terminus amine group of peptides, even in the presence of lysine residues, ensuring precise and selective modification.
Implications for Research and Therapeutics
The ability to selectively modify peptides at the N-terminus opens up a wide range of possibilities for labeling diverse peptides and larger proteins for various purposes, such as purification, detection, and therapeutic applications. The research team is currently evaluating the biological activity of peptides modified through this new reaction and aims to extend its application to larger peptides, such as proteins and antibodies, with the potential for significant advancements in drug delivery.
Overall, the development of this site-selective dual modification protocol represents a significant advancement in the field of peptide research and holds great promise for future research and therapeutic applications.

Leave a Reply