In today’s world, where the issues of waste management and environmental pollution are increasingly critical, researchers at Case Western Reserve University have taken up the challenge of developing innovative solutions. They are currently working on ways to convert waste into fuels and valuable chemicals using energy-efficient processes powered by renewable sources. One particular challenge they are close to overcoming is the conversion of carbon dioxide (CO2), a major greenhouse gas, into valuable chemicals using electricity. This groundbreaking research has the potential to transform how we address waste management and contribute to a sustainable future.
The conversion of CO2 into chemical compounds and fuels is not a simple task. Converting CO2 requires high pressures, high temperatures, and special materials. In the quest to find a solution, previous research has primarily focused on developing catalyst materials and understanding the energy-intensive CO2 conversion reaction in water-based electrolytes. However, water-based systems have limited capacity for CO2 and often result in unwanted side reactions, like hydrogen gas emissions.
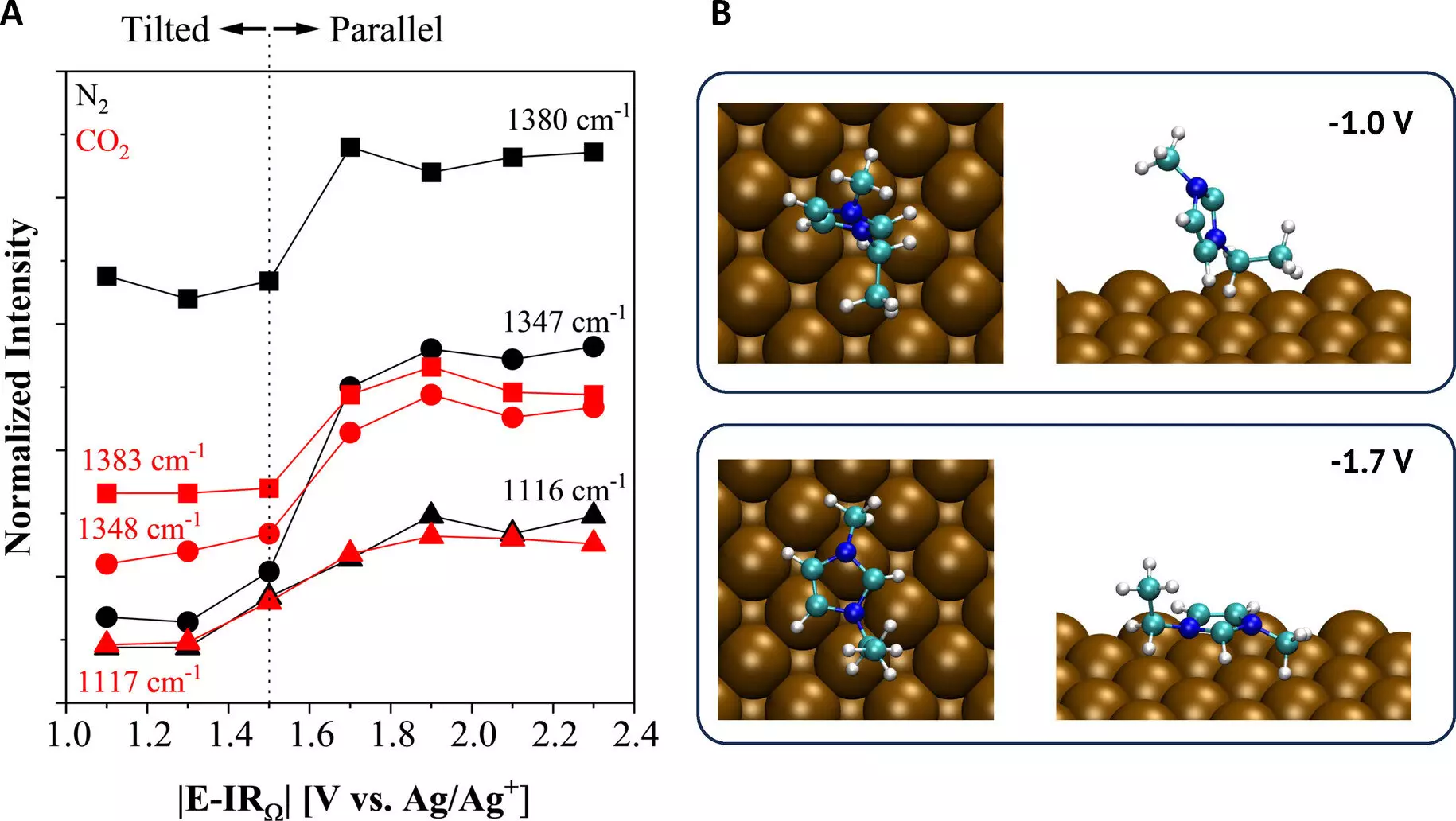
A recent study published in the European journal Angewandte Chemie by researchers at Case Western Reserve University presents a breakthrough in CO2 conversion using ionic liquids. Ionic liquids are salts that have the unique property of melting below 100°C. The research team developed ionic liquids that are liquid at room temperature, which enables their practical application. These ionic liquids also possess a high capacity for CO2 capture and maintain electrochemical stability. This means that the electrochemical process can be achieved effectively, without the unwanted side products found in traditional electrolysis.
The study, led by Oguz Kagan Coskun, a doctoral student in the research group, utilized spectroscopic and electroanalytical techniques to uncover the fundamental mechanisms that allow ionic liquids to activate the CO2 reduction reaction at the copper electrode surface. The team demonstrated that less energy is required to drive the reaction, opening up possibilities for the creation of a variety of industrially relevant products. This advancement in CO2 conversion technology holds immense potential for reducing greenhouse gas emissions and providing sustainable alternatives to traditional fuel sources.
The findings of the study provide crucial insights into the properties of the reaction environment, specifically concerning unconventional electrolytes. This knowledge deepens our understanding of the electrochemical processes involved in CO2 conversion and paves the way for further advancements in catalyst design. By gaining better control over the chemicals produced during the reaction, researchers can optimize the outcome and maximize the efficiency of CO2 recycling.
With a clear path ahead, the research team at Case Western Reserve University aims to explore the individual reaction steps in greater detail. This deeper understanding will inform subsequent electrolyte designs and contribute to the advancement of electrochemical approaches to CO2 recycling. Their ultimate goal is to develop technologies that can efficiently capture CO2 from waste, or even directly from the air, and convert it into valuable products under benign conditions.
The advancements made by the researchers at Case Western Reserve University in waste-to-fuel conversion technology mark a significant step forward in addressing pressing environmental concerns. The use of ionic liquids and electrochemical processes offers a promising pathway to convert carbon dioxide, a major greenhouse gas, into valuable chemicals. By harnessing renewable sources of energy and developing energy-efficient processes, we can minimize our reliance on traditional fuel sources and contribute to a more sustainable future. As these technologies continue to evolve, we can expect to see significant progress in waste management and a reduction in greenhouse gas emissions. The potential for a greener and cleaner future is within reach.

Leave a Reply